LB Se PAA Horse Feed Experiment
Chlorine is a dominant signal
It looks like Cl-35 is abundant as you see photon energies of 146 keV and 2127 keV (you can barely see 1176 keV) from Cl-34's decay (neutron knocked out of Cl-35).
The half life is 32 minutes.
Should check the half life from the run AccOnAlInDetASe-AinDetD_001.root using the calibration
MPA->Draw("0.18063+0.960133*evt.Chan>> SeRun_008(8000,0.5,8000.5)","evt.ADCid==3");
Irradiation of Horse Mineral Supplement
Below is the EMSL report for the horse feed sample. https://wiki.iac.isu.edu/index.php/File:EMSL_Report_Horse_Feed.pdf
First, look at the peak around 146 keV
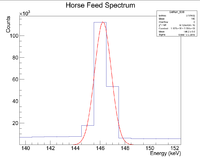
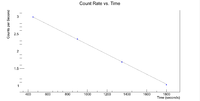
Next I plotted the counts as a function of time to get an exponentially decaying graph. When doing an exponential fit here, the parameter "b" given by root will be the decay constant.
Root gives a half life of 32.9508 +/- 0.01 minutes
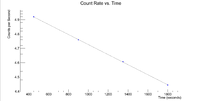
Now do the same for the 2127 keV line
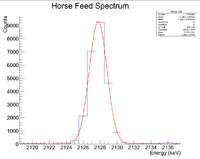
Here are the counts plotted as a function of time
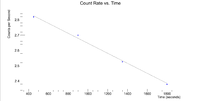
Root gives a half life of 35.3962 +/- 0.2 minutes
Potassium is a potential signal
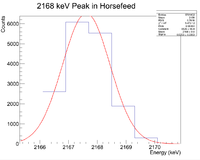
Looking at the spectrum for the fast irradiation sample, there are 2 prominent lines that could be from 38-K. The mechanism would be a single neutron knockout from a stable 39-K nucleus. The two most dominant energies of the three for 38-K are 2167 keV and 3936 keV and the half life is 7.63 minutes. Below is a fit to the energy spectrum histogram
Now check the half life
Root gives a half life of 8.03 +/- 0.02 minutes
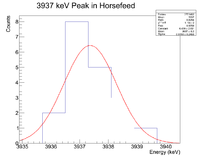
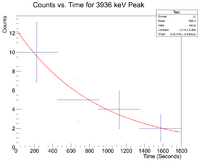
Next check the 3936 peak
and check the half life
Root gives a value for b = - 1.14372x10^(-3), which in turn gives a half life of 10.1 minutes
It seems very possible that 38-K could be in the sample of horse feed.