Forest Scintillators
- Scintillation
- the process by which atoms or molecules of a material are given enough energy by an incident particle of radiation to "excite" the system where upon its relaxation to a lower energy state is accomplished through the emission of light.
In general, most materials are capable of scintillating.
It takes about s ( 1 ns) for an atom to de-excite by giving off light. Atoms can , however, loose there energy by colliding with other atoms. Atoms which collide on time scales less than 1 ns could loose their energy via the collision instead of through the emmission of light.
At standard temperature and pressure, air has a velocity of about
and a mean free path (distance between collisions) of 0.3 nm
this means the time between air molecule collision is about
This air can loose its energy via collisions instead of through the emmision of light.
The situation for your average solid isn't much better
An atom in a solid can collide with 10 other atoms before loosing energy by emmitting a photon.
Organic Scintillators
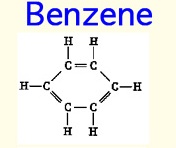
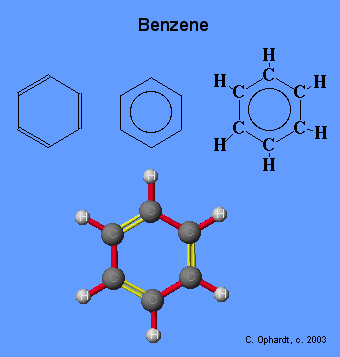
Benzene/Toluene
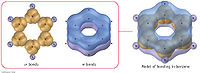
Benzene, and it's less toxic cousin Toluene, is the magic material for producing scintillation light. The electrons on a Benzene ring are so loosely bound (de-localized) that they do not feel atomic collisions.
Vibration state
Having the atom de-excite through the emission of photons is just half of the problem. Getting the photons out of the material to a photon detector is the last half of the problem. Fortunately , there are vibrational states which allows atom to de-excite produce photons which are not able to excite the next Benzene ring the pass by. This makes the material transparent to the scintillation light.
In-organic scintillators
While organic scintillators rely on de-localize -orbitals to emmit light, Inorganic scintillators rely on the electronic band structure of crystals. The physical process involves the creation of electron - hole pairs to create light.
NaI is an example of such a scintillator.
The Na in the crystal has a 3S electron conduction band
The I in the crystal has a 5P hole valence band
If you deposit 5.8 eV into the crystal you create a electron-hole pair which can then recombine and emitt the energy in the form of light.
The catch is that this only happens if the temperature is less than 300 K, otherwise the energy from recombination will go into vibrational (phonon) energy.
This means that pure NaI is a bad scintillator at room temperature.
If you mix in some Thalium (dopant), then the electron-hole (hole halide) ion will more readily get trapped by the Thalium and emitt light.