Difference between revisions of "Forest ModernPhysics"
| Line 59: | Line 59: | ||
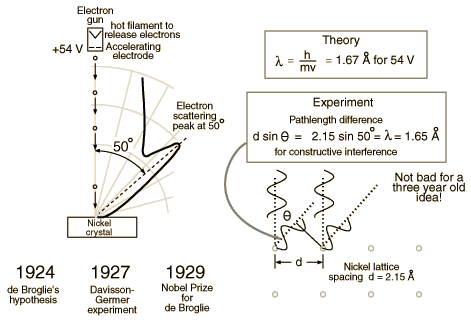
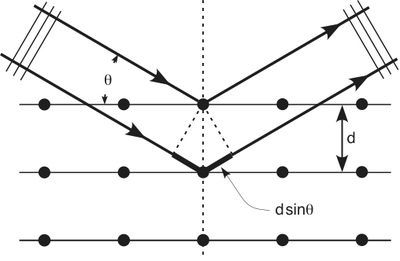
| − | + | Clinton Davisson and Lester Germer in 1927 published conclusive evidence for the diffraction of electron waves using 54 eV electrons impinging a crystal made of nickel. | |
One problem to overcome for the experiment was that such a low energy electron scatters in air. The had to do the experiment in a vacuum. | One problem to overcome for the experiment was that such a low energy electron scatters in air. The had to do the experiment in a vacuum. | ||
Revision as of 02:55, 30 September 2009
Matter Waves (Wave Particle Duality)
Special relativity said that
if m=0
Plank said he could fit the Black Body radiation data assuming that that
where = Plank's constant
Combining the two we have
photons have momentum like a particle (mv)
Do particles reciprocate and behave like photons?
De Broglie's Hypothesis
If photons can behave like particles by having momentum
Then can a particle behave like a wave by having wavelength
or
de Broglie Hypothesis
Davisson and Germer
We know that X-rays having a wavelength of make a diffraction pattern on an aluminum foil.
Another way to calculate
- What would be the energy of an electron with the same wavelength as the above X-ray?
relativistic total energy relation
- = 511.3 keV
relativistic kinetic energy
- Note classical physics may be used for electrons below 50 keV
Clinton Davisson and Lester Germer in 1927 published conclusive evidence for the diffraction of electron waves using 54 eV electrons impinging a crystal made of nickel.
One problem to overcome for the experiment was that such a low energy electron scatters in air. The had to do the experiment in a vacuum.
From hyperphysics: