Difference between revisions of "ArCO2 IonizationPhysics"
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
| + | A Min Ion Particle traversing Ar gas at 1 atm ionizes 22 atoms/cm Primary and 94-100 Total per cm. The Primary is an ionization from the incident particle, Total includes ionization from secondaries. | ||
| + | |||
<math>\bar{E}=</math> 27 eV = average energy to ionize an electron in an Argon Atom | <math>\bar{E}=</math> 27 eV = average energy to ionize an electron in an Argon Atom | ||
| Line 16: | Line 18: | ||
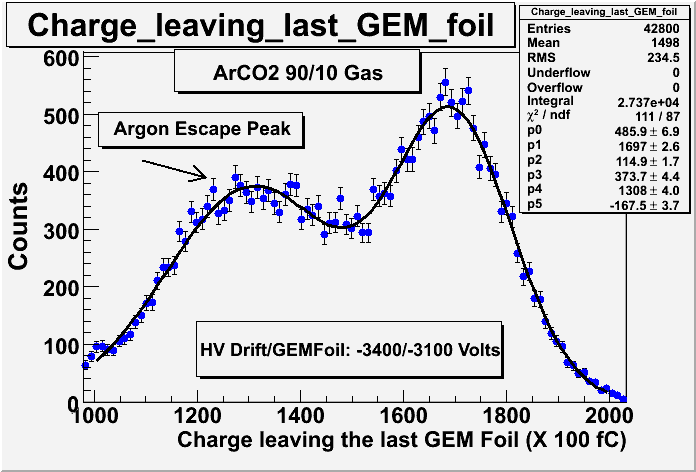
:You need 3.203 keV to ionize a K-shell electron in Argon. If your incident ionizing particle (photon or electron) has more than that energy then it is possible to excite Argon so it becomes a source of photons (X-rays) during the ionization process. The Ar-Ka (2.958 keV) X-ray is one likely X-ray. If that photon ESCAPEs the detector without causing ionization, then your signal will contain less ionized electrons. The process is such that during the ionizing of multiple Argon atoms by a photon loosing energy to the gas, the photon will excite one Argon atom such that it emits an X-ray which excapes the chamber. | :You need 3.203 keV to ionize a K-shell electron in Argon. If your incident ionizing particle (photon or electron) has more than that energy then it is possible to excite Argon so it becomes a source of photons (X-rays) during the ionization process. The Ar-Ka (2.958 keV) X-ray is one likely X-ray. If that photon ESCAPEs the detector without causing ionization, then your signal will contain less ionized electrons. The process is such that during the ionizing of multiple Argon atoms by a photon loosing energy to the gas, the photon will excite one Argon atom such that it emits an X-ray which excapes the chamber. | ||
| + | |||
| + | |||
| + | [[File:FirstQweakData_HV3400Volts_ChargeLeavingLastGEMFoil_4-04-09-Datafile_r877.gif]] | ||
Latest revision as of 19:07, 21 March 2011
A Min Ion Particle traversing Ar gas at 1 atm ionizes 22 atoms/cm Primary and 94-100 Total per cm. The Primary is an ionization from the incident particle, Total includes ionization from secondaries.
27 eV = average energy to ionize an electron in an Argon Atom
average number of photoelectrons produced
Quenching Gas:
1.) reduces the influence of the positive ions creates on the photoelectron signal: The excited Ar+ atoms emit photon in the UV range which are absorbed by the quenching gas
2.)Collisions with the quenching gas will neutralize the Ar+ ions. When the quenched gas, having an electron remove by the Ar+ collision, reaches the cathode and collects an electron, most of the energy goes into dissociation of the Quench gas.
If the quech gas is CH4 then
CH4+ H2 + CH2
- Argon Escape peak
- You need 3.203 keV to ionize a K-shell electron in Argon. If your incident ionizing particle (photon or electron) has more than that energy then it is possible to excite Argon so it becomes a source of photons (X-rays) during the ionization process. The Ar-Ka (2.958 keV) X-ray is one likely X-ray. If that photon ESCAPEs the detector without causing ionization, then your signal will contain less ionized electrons. The process is such that during the ionizing of multiple Argon atoms by a photon loosing energy to the gas, the photon will excite one Argon atom such that it emits an X-ray which excapes the chamber.