PAA Selenium
Using PAA ro measure Selenium concentrations.
According to Krouse<ref name="Krous1962"> H.R. Krause and H.G. Thode,"Thermodynamic Properties and Geochemistry of Iosotopic Compounds of Selenium",.Can. J. Chem., vol 40, pg 367</ref> , the fractional concentration of Se-82/Se-76 in plant material is observed to be less than from primordial (meteoric) concentrations by as much as 1.2%. Anaerobic bacteria are known to reduce selenates and senelites in biological systems. This may be the reason plant material has fractionation of selenium isotopes. They also observe excess concentrations of up to 0.4% in soil.
Plant material appears to detect environmental selenium.
Can one use plant material to measure the provenance of selenium?
Can one perform PAA measurements of Se-82 and Se-76?
Neutron knockout of Se-82
If you knock a neutron out of Se-82 you produce the unstable isotope Se-81 which Beta emitts with half life of 18 min and a meta-state that emmits a 103 keV gamma with a 57 minute half life.
Other prominent photons
260 & 276 keV for the 57 minute half life isotope
Neutron knockout of Se-76
If you knock a neutron out of Se-76 you produce the unstable isotope Se-75 which has a half life of 119 days.
The prominent photons emitted have the following energies
136, 264, and 279 keV
The article below describes how plant material and soil contain Se-76 to Se-82 ratios that differ from other natural samples by 1.5%. They argue that it is due to the bacteria living in plant material.
File:Krouse CanJournChem 40 1962 p367.pdf
Plant material is a natural way to sample the selenium content to determine if there are difference isotopic ratios due to the impact of human activities on the environment.
Experiments
Chlorine
It looks like Cl-35 is abundant as you see photon energies of 146 keV and 2127 keV (you can barely see 1176 keV) from Cl-34's decay (neutron knocked out of Cl-35).
The half life is 32 minutes.
Should check the half life from the run AccOnAlInDetASe-AinDetD_001.root using the calibration
MPA->Draw("0.18063+0.960133*evt.Chan>> SeRun_008(8000,0.5,8000.5)","evt.ADCid==3");
Irradiation of Horse Mineral Supplement
Chlorine is a dominant signal
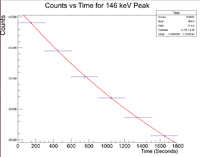
First, look at the peak around 146 keV
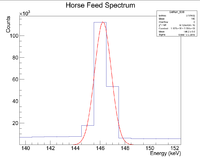
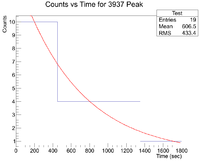
Next I plotted the counts as a function of time to get an exponentially decaying graph. When doing an exponential fit here, the parameter "b" given by root will be the decay constant.
Root gives a value b = 3.59543x10^-4, which yields a half life of 32.1 minutes.
Now do the same for the 2127 keV line
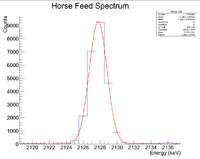
Here are the counts plotted as a function of time
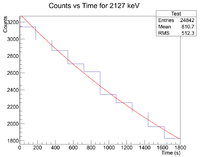
Root gives b = 3.31508x10^-4, which yields a half life of 34.8 minutes.
Potassium is a potential signal
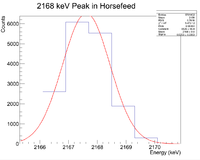
Looking at the spectrum for the fast irradiation sample, there are 2 prominent lines that could be from 38-K. The mechanism would be a single neutron knockout from a stable 39-K nucleus. The two most dominant energies of the three for 38-K are 2167 keV and 3936 keV and the half life is 7.63 minutes. Below is a fit to the energy spectrum histogram
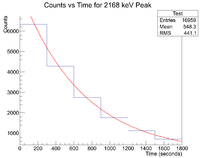
Now check the half life
Root gives a value for b = -1.44513x10^(-3), which in turn gives a half life of 8 minutes
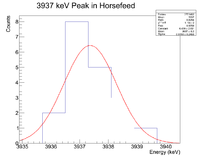
Next check the 3936 peak
and check the half life
Root gives a value for b = - 1.52044x10^(-3), which in turn gives a half life of 7.6 minutes
It seems very possible that 38-K could be in the sample of horse feed.
Possible Yb-167 in the Sample
Yb-167 has a half life of 17.5 minutes and can be produced by knocking a neutron out of Yb-168, which is stable.
I looked at the horse feed spectrum and compared the spectral lines for Yb-167, below is a table of those results:
| Energy (keV) | Spectrum Energy (keV) | Half life (min) |
| 90.84 | 89 | 18.23 |
| 106.18 | 105 | 18.0 |
| 112.88 | 113 | 18.86 |
| 132.02 | 137 | 19.2 |
| 161.29 | 161 | 17.3 |
| 203.75 | 204 | 17.27 |
| 280.5 | 281 | 17.0 |
| 290.89 | 289 | 16.7 |
| 323.5 | 322 | 16.6 |
| 354.0 | 354 | 16.7 |
| 375.9 | 378 | 15.5 |
| 387.0 | 388.4 | 19.1 |
| 398.1 | 402 | 16.1 |
| 688.5 | 691 | 15.6 |
| 733.2 | 736 | 17.1 |
| 920.32 | 922 | 16.6 |
| 977.9 | 980 | 16.9 |
| 1025.9 | 1028 | 16.9 |
| 1242 | 1245 | 17.5 |
| 1340.1 | 1341 | 16.5 |
| 1410.4 | 1413 | 18.6 |
| 1433.4 | 1437 | 16 |
| 1631.7 | 1630 | 19.6 |
The average of these half lives is 17.22 minutes, but it turns out this was just coincidental noise meaning we must overbin the spectrum.
Investigation of Se_B_002
Using the Se_B_002, I will investigate the peaks seen in the spectrum.
The first peak seen in the spectrum was at 74.1 \%pm%\ 2.947 keV. The half life was found to be 324.94 days. When doing the rad search I used a window from 69-79 keV and a half life window from 200-400 days.
| Isotope | Energy (keV) | I(%) | Half life |
| 153-Gd | 69.67 | 2.419 | 240 days |
| 254-Es | 69.7 | not given | 275.7 days |
| 254-Es | 70.4 | not given | 275.7 days |
| 153-Gd | 75.4 | 0.0783 | 240.4 days |
254-Es doesn't really have a mechanism to produce this isotope. The nearest stable isotope would have to have greater than a quadruple neutron knockout, or a triple proton knockout.
153-Gd could possibly be produce from 154-Gd by knocking out a neutron, but the half life is still far off. The hottest decay lines for 153-Gd are 97.4 and 103.2 keV with I = 29 and I = 21.11 respectively. Neither of these lines are seen in the spectrum so it is unlikely that it is either of these isotopes.
The next clear peak is seen at 85.5 \%pm%\ 3.89 keV. The half life was found to be 99.1 days. When doing the rad search I set a range of energies from 75-95 keV with a half life range from 50-150 days. The possible isotope information is below
| Isotope | Energy (keV) | Ig(%) | Half life |
| 257-Fm | 75.0 | 0.200 | 100.5 days |
| 174m-Lu | 76.47 | 0.0638 | 142 days |
| 170-Tm | 78.63 | 0.00347 | 128.6 days |
| 159-Dy | 79.45 | 0.00048 | 144.4 days |
| 168-Tm | 79.804 | 10.53 | 93.1 days |
| 258-Md | 80.1 | 2.43 | 51.5 days |
| 257-Fm | 80.2 | 0.085 | 100.5 days |
| 75-Se | 80.94 | 0.0077 | 119.779 days |
| 183-Re | 82.9182 | 0.294 | 70.0 days |
| 170-Tm | 85.25474 | 2.5 | 128.6 days |
| 182-Ta | 84.6802 | 2.65 | 114.43 days |
| 183-Re | 84.7125 | 0.97 | 70.0 days |
| 188-W | 85.32 | 0.0024 | 69.4 days |
| 160-Tb | 86.7882 | 13.15 | 72.3 days |
| 258-Md | 86.9 | 0.5615 | 51.5 days |
| 168-Tm | 87.73 | 0.000016 | 93.1 days |
| 127m-Te | 88.26 | 0.084 | 109 days |
| 123m-Te | 88.46 | 0.092 | 119.7 days |
| 175-Hf | 89.36 | 2.4 | 70 days |
| 258-Md | 91.0 | 0.3018 | 51.5 |
| 148-Eu | 92.6 | 0.019 | 54.5 days |
| 151-Gd | 93.21 | 0.0019 | 124 days |
| 160-Tb | 93.919 | 0.0566 | 72.3 days |
The following isotopes have mechanisms that are larger than a 2 neutron/proton knockout and larger than 1n1p knockout: 257-Fm, 258-Md, 148-Eu.
First Observation of Se lines
Using the 44 Machine at 7 kW power and 44 meV incident electron energy to produce a bremsstrahlung spectrum with a mean energy of 15 meV.
All runs lasting less than 214 seconds have time stamp that gives real time if you divide by clock frequency of 20 MHz. The first 32 bits are used for a real time measurement.
Detector Efficiency
Below is the runlist for finding the efficiency of the detector at position R
| Source | Serial # | Reference Date | Activity | Start | Stop | Live |
| Na-22 | 129743 | 7-01-08 | 9.427 microCi | 15:49 | 16:19 | 1796.803 |
| Cs-137 | 129793 | 7-01-08 | 1.006 microCi | 14:25 | 14:56 | 1879.606 |
| Mn-54 | 129807 | 7-01-08 | 11.77 microCi | 15:00 | 15:30 | 1793.420 |
| Co-60 | 129740 | 7-01-08 | 10.42 microCi | 15:33 | 15:43 | 569.725 |
Below are the theoretical calculations for the theoretical decay frequencies
Na-22, 9.427micro Ci on July 1, 2008, half life 2.602 +/- 0.002 years, 99.937% for 1274.52 and 178.8 for 511 line , activity in March 31, 2016 =1.196micro Ci
- = for the 1274 line
- = for the 511 line
Cs-137, 661.660 line, 85.21% * 1.066micro Ci on July 1, 2008, half life 30.0 +/- 0.2 yrs, March 31, 2016 activity = 0.891micro Ci expected rate for 661 line
- =
Mn-54, on July 1, 2008, half life =312.20 +/- 0.07 days, 99.975% intensity on 834.826 , March 31, 2016 activity =0.02328micro Ci
- = for the 834 line
Co-60, 10.42micro Ci July 1, 2008, half life 5.271 +/- 0.001 years, 99.0 % for 1173.237 and 99.9824 % for 1332.501, March 31, 2016 activity=3.759micro Ci
- = for the 1173 line
- = for the 1332 line
Below is a runlist for position k
| Source | Serial # | Reference Date | Activity | Start | Stop | Live |
| Na-22 | 129743 | 7-01-08 | 9.427 microCi | 14:54 | 15:01 | 434.087 |
| Cs-137 | 129793 | 7-01-08 | 1.006 microCi | 15:48 | 15:55 | 413.925 |
| Mn-54 | 129807 | 7-01-08 | 11.77 microCi | 15:28 | 15:40 | 705.186 |
| Co-60 | 129740 | 7-01-08 | 10.42 microCi | 15:41 | 15:47 | 346.092 |
Below are the theoretical decay frequencies
Na-22, 9.427micro Ci on July 1, 2008, half life 2.602 +/- 0.002 years, 99.937% for 1274.52 and 178.8 for 511 line , activity in April 14, 2016 =1.183micro Ci
- = for the 1274 line
- = for the 511 line
Cs-137, 661.660 line, 85.21% * 1.066micro Ci on July 1, 2008, half life 30.0 +/- 0.2 yrs, April 14, 2016 activity =0.890 micro Ci expected rate for 661 line
- =
Mn-54, on July 1, 2008, half life =312.20 +/- 0.07 days, 99.975% intensity on 834.826 , April 14, 2016 activity =0.02251micro Ci
- = for the 834 line
Co-60, 10.42micro Ci July 1, 2008, half life 5.271 +/- 0.001 years, 99.0 % for 1173.237 and 99.9824 % for 1332.501, April 14, 2016 activity=3.74micro Ci
- = for the 1173 line
- = for the 1332 line
References
<references/>
MSDS
Selenium shot, amorphous, 2-6 mm, Puratronic, 99.999% Alfa Aesar product # 10603 File:AlphaAesarSelenium MDSD.pdf
Informative links
https://inldigitallibrary.inl.gov/sti/3169894.pdf