Difference between revisions of "TF EIM Chapt1"
| Line 1: | Line 1: | ||
| + | = Fundamentals= | ||
| + | ==Charge== | ||
| + | |||
| + | Every stable and independent object (particle) that has charge has been observed to contain a quantized unit of charge which is a multiple of <math>1.6 \times 10^{-19} Coulombs</math> | ||
| + | |||
| + | What are the obervations/experiments? | ||
| + | |||
| + | Experiment 1: Matter is composed of Atoms with a positively charged nucleus surround by negatively charged electrons. If we now the charge of one mole of electrons (<math>F</math>= Faradays constant) and the number of electrons in a mole (<math>N_A</math> = Avagadros number)then the charge of a single electron is given by | ||
| + | |||
| + | :<math>e = \frac{F}{N_A}</math> | ||
| + | |||
| + | Experiment 2: Oil drop experiment | ||
| + | |||
| + | Experiment 3: The Hall Effect and the Josephson Effect | ||
| + | |||
| + | ==Electric Field== | ||
| + | |||
| + | Two charged object separated by a distance (D) will feel a force between them known as the coulomb force. The magnitude of this force has been experimentally shown to be | ||
| + | |||
| + | ; <math>\left | \vec{F}_{coul} \right | = \frac{1}{4 \pi \epsilon_0} \frac{q_1 q_2}{r^2}</math> | ||
| + | |||
| + | where | ||
| + | :<math> \varepsilon_0 =\frac {1}{\mu_0 c_0^2}=8.854187817 \times 10^{-12}</math>F/m | ||
| + | = a experimentally measured quantity satisfying the above relationship know as the permittivity of free space. | ||
| + | |||
| + | This force may be described in terms of an electric field E such that | ||
| + | |||
| + | :<math>\vec{E} = \frac{\vec{F_q}}{q}</math> | ||
| + | |||
| + | |||
| + | Where | ||
| + | |||
| + | ;F= force between the objects | ||
| + | |||
| + | A separated object of finite charge creates an electric field. | ||
| + | |||
| + | ==Electric potential== | ||
| + | |||
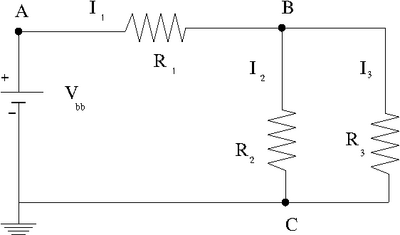
=Ohm's Law= | =Ohm's Law= | ||
| Line 8: | Line 46: | ||
==Voltage== | ==Voltage== | ||
| + | |||
| + | The MKS unit for Voltage is a Joule per Coulomb <math>\left ( \frac{\mbox{J}}{\mbox{C}}\right )</math> | ||
| + | |||
| + | Voltage in circuits is typically defined as the electric potential energy per unit charge relative to ground. | ||
| + | |||
==Current== | ==Current== | ||
==Resistance== | ==Resistance== | ||
Revision as of 17:40, 31 December 2010
Fundamentals
Charge
Every stable and independent object (particle) that has charge has been observed to contain a quantized unit of charge which is a multiple of
What are the obervations/experiments?
Experiment 1: Matter is composed of Atoms with a positively charged nucleus surround by negatively charged electrons. If we now the charge of one mole of electrons (= Faradays constant) and the number of electrons in a mole ( = Avagadros number)then the charge of a single electron is given by
Experiment 2: Oil drop experiment
Experiment 3: The Hall Effect and the Josephson Effect
Electric Field
Two charged object separated by a distance (D) will feel a force between them known as the coulomb force. The magnitude of this force has been experimentally shown to be
where
- F/m
= a experimentally measured quantity satisfying the above relationship know as the permittivity of free space.
This force may be described in terms of an electric field E such that
Where
- F= force between the objects
A separated object of finite charge creates an electric field.
Electric potential
Ohm's Law
- resistance is a constant
- = constant
Voltage
The MKS unit for Voltage is a Joule per Coulomb
Voltage in circuits is typically defined as the electric potential energy per unit charge relative to ground.