Difference between revisions of "TF EIM Chapt1"
| Line 120: | Line 120: | ||
<math>W = \int_A^B \vec{F} \cdot d\vec{r} </math> Potential Energy | <math>W = \int_A^B \vec{F} \cdot d\vec{r} </math> Potential Energy | ||
| − | :<math>=\int_A^B q (-\vec{E}) \cdot d\vec{r} </math> | + | :<math>=\int_A^B q (-\vec{E}) \cdot d\vec{r} =-\int_A^B q \vec{E} \cdot d\vec{r} </math> |
<math>(-\vec{E} \cdot d\vec{r}) = \left | \vec{E} \right | dr</math> : The applied force is directed opposite to the Electric field but in the same direction as the displacement<math> \vec{r}</math> | <math>(-\vec{E} \cdot d\vec{r}) = \left | \vec{E} \right | dr</math> : The applied force is directed opposite to the Electric field but in the same direction as the displacement<math> \vec{r}</math> | ||
Revision as of 21:43, 11 January 2011
Fundamentals
Charge
Every stable and independent object (particle) that has charge has been observed to contain a quantized unit of charge which is a multiple of
What are the obervations/experiments?
Experiment 1: Matter is composed of Atoms with a positively charged nucleus surround by negatively charged electrons. If we now the charge of one mole of electrons (= Faradays constant) and the number of electrons in a mole ( = Avagadros number)then the charge of a single electron is given by
Experiment 2: Oil drop experiment
http://www.youtube.com/watch?v=XMfYHag7Liw&feature=player_embedded
Experiment 3: The Hall Effect and the Josephson Effect
- Coulomb
- The amount of charge that flows through any cross section of a wire in 1 second if there is a steady current of 1 ampere in the wire.
Coulomb Force
Two charged object separated by a distance (d) will feel a force between them known as the coulomb force. The magnitude of this force has been experimentally shown to be
where
- F/m = a experimentally measured quantity satisfying the above relationship know as the permittivity of free space.
- charge of first object
- charge of second object
- distance between the charges
(Charles Augustin Coulomb first measured the attract and repulsive force between two charged objects using a torsion balance around 1785)
This force may be described in terms of an electric field E such that
Where
- F= force between the objects
Electric Field
Vector Field
- What is a vector field?
- A vector field is the association of each point in space with a direction (vector).
Example: Gravity. On the surface of the earth you observe that an apple will fall down to the ground. You can measure the magnitude of this force by observing the attraction much like how coulomb observed how two charges attract or repel each other. You can define a vector ( which points directly down and tell you the direction that an apple will accelerate if it is released. If you construct such that
- = acceleration of an apple towards the earth's surface when close to the earth's surface.
Where G= gravitational constant =
Electric Field Vector
| Electric Field | Grass seeds in Insulating Liquid |
Instead of an apple of mass we can use a test charge of charge .
Instead an earth of mass we can use an object of charge .
We now define an Electric field Vector such that
- ;Notice the direction tells you which way a positive charge will move
- Note
- The definition of requires that the magnitude of the test charge approach zero, because the test charge can disturb the electric field we are actually trying to determine.
Lines of Coulomb Force
Once you know the Electric Vector Field you know the force on any charge placed in that field simply by
In other words, the tangent to the direction of the Force vector indicates the direction of the vector field .
- Unfortunately, charge can be either positive or negative whereas mass is always a positive quantity so the above uses the convention of a positive (test) charge.
Electric potential
The Electric potential (V) is a scalar which may be used to describe Electric Fields as well.
A charge which moves along an electric field line is having work done on it by an electric field.
Electric Potential Energy
From the work energy theorem, Work is equal to the change in energy of a system
- Energy
The work done moving a change along an electric field line is
Potential Energy
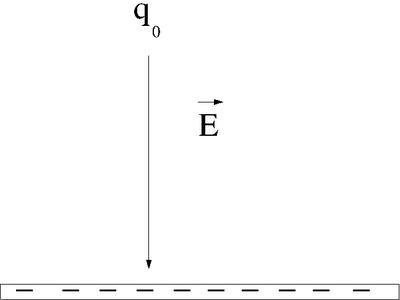
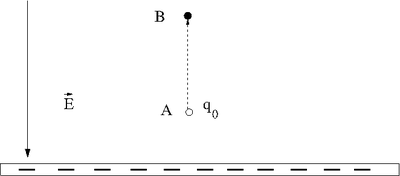
Let's say you lift a unit of POSITIVE charge (q) from location A above a sheet of negative charge to a higher location B.
- Note
- This is just like the gravitational example of walking up a flight of stairs.
You will need to apply a force on the positive charge to lift it up because the negatively charge sheet is attracting it (like the earth attracts your mass as you walk up a flight of stairs). For simplicity, let's assume you move at a constant velocity or no acceleration. In other words the attractive coulomb force is balanced by the force you use to push the positive charge to a higher location above the negatively charged infinite sheet.
Potential Energy
: The applied force is directed opposite to the Electric field but in the same direction as the displacement
- = Potential Energy Difference
- Note
- The work done by the electric field is negative (the force from the electric field is opposite to the direction of motion).
Voltage (Electric Potential)
The Electric Potential Difference
The change in the potential depends on the sign of q_0
Positive (Negative) charge naturally goes from higher (lower) potential to lower (higher) voltage.
Voltage decreases(increases) when you travel parallel(anti-parallel) to the electric field.
When you measure the electric potential (Voltage) you are actually measuring a potential difference V with respect to some point that is customarily chosen to be zero.
Remember this when you place the black probe of your voltmeter onto some conducting point. The black probe might not be at "ground".
If you define the potential to be zero and move the charge a distance then the voltage is given by
- V = V_B = Ed
Electromotive Force(emf)
An electromotive force is used to describe the external work expended per unit of charge to produce an electric potential difference across two open-circuited terminals.
Battery
A battery uses a chemical reaction to separate atoms into ions and push electrons onto the negative terminal. The terminal of a battery are usually made out of two different metal electrodes separated by a solution/paste containing ions called the "electrolyte".
Ex: Carbon and zinc electrodes separated by ammonium chloride results in a chemical reaction which generates a 1.5 Volt potential difference between the carbon and zinc electrodes. This is called a cell. From cells you can make batteries by tying cells in series or parallel.
| Bat. Type | Cell emf (V) | characteristic |
| Carbon-Zinc | 1.5 | flashlight battery, voltage drops slowly with time |
| Alkaline-Maganeses | 1.5 | long lasting lasting flashlight battery, high current capacity, voltage drops gradually |
| Nickel-cadmium | 1.25 | rechargeable flashlight battery |
| Lead-acid | 2.6 | car battery, high currents, rechargeable, sufluric acid electrolyte |
| Silver Oxide | 1.5 | hearing aids and wathes, low current, constant voltage |
| Mercury | 1.35 | sudden voltage drops, indoor equipment. |
Notice the battery is an isolated voltage source. This means the definition of zero potential can be made at any one point in a circuit with one battery. Because the battery is using a chemical reaction, the electrons come from inside the battery and are not pulled or pushed from the earth (a ground).
As soon as you use the battery the emf changes and is called a voltage because of the internal resistance of the battery (see Lab 1). The voltage rating of the battery represents its "open-circuit" voltage; the voltage when it is not connected.
Unfortunately, batteries have an internal voltage which is only negligible if the external circuits resistance is much larger then the battery's internal resistance. When a battery is "dead" its internal resistance has become equivalent to the circuit the battery is trying to drive (flashlight). The chemical reaction has ceased to effectively pump electrons onto the terminal and as a result "resists" current flow.
Ohm's Law
- resistance is a constant
- = constant
Voltage
The MKS unit for Voltage is a Joule per Coulomb
Voltage in circuits is typically defined as the electric potential energy per unit charge relative to ground.