Difference between revisions of "TF EIM Chapt1"
| Line 16: | Line 16: | ||
==Electric Field== | ==Electric Field== | ||
| − | Two charged object separated by a distance ( | + | Two charged object separated by a distance (d) will feel a force between them known as the coulomb force. The magnitude of this force has been experimentally shown to be |
; <math>\left | \vec{F}_{coul} \right | = \frac{1}{4 \pi \epsilon_0} \frac{q_1 q_2}{r^2}</math> | ; <math>\left | \vec{F}_{coul} \right | = \frac{1}{4 \pi \epsilon_0} \frac{q_1 q_2}{r^2}</math> | ||
Revision as of 16:09, 3 January 2011
Fundamentals
Charge
Every stable and independent object (particle) that has charge has been observed to contain a quantized unit of charge which is a multiple of
What are the obervations/experiments?
Experiment 1: Matter is composed of Atoms with a positively charged nucleus surround by negatively charged electrons. If we now the charge of one mole of electrons (= Faradays constant) and the number of electrons in a mole ( = Avagadros number)then the charge of a single electron is given by
Experiment 2: Oil drop experiment
Experiment 3: The Hall Effect and the Josephson Effect
Electric Field
Two charged object separated by a distance (d) will feel a force between them known as the coulomb force. The magnitude of this force has been experimentally shown to be
where
- F/m
= a experimentally measured quantity satisfying the above relationship know as the permittivity of free space.
This force may be described in terms of an electric field E such that
Where
- F= force between the objects
A separated object of finite charge creates an electric field.
Electric potential
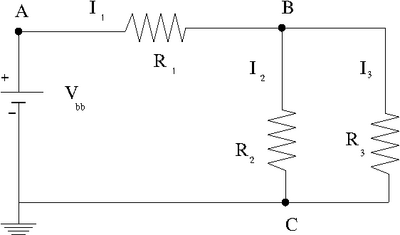
Ohm's Law
- resistance is a constant
- = constant
Voltage
The MKS unit for Voltage is a Joule per Coulomb
Voltage in circuits is typically defined as the electric potential energy per unit charge relative to ground.