Difference between revisions of "Finding the Space Between Lead Shots"
(→Plan 2) |
|||
| (5 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | [http://wiki.iac.isu.edu/index.php/PhotoFission_with_Polarized_Photons_from_HRRL Go Back] | ||
| + | |||
| + | |||
==Plan 1== | ==Plan 1== | ||
By Mass | By Mass | ||
| Line 65: | Line 68: | ||
V<sub>Pb</sub> = (m<sub>4</sub> - m<sub>1</sub>)/ρ<sub>H2O</sub>=5896.8 <math>cm^3</math> | V<sub>Pb</sub> = (m<sub>4</sub> - m<sub>1</sub>)/ρ<sub>H2O</sub>=5896.8 <math>cm^3</math> | ||
| − | Ratio = V<sub>Pb</sub>/V<sub> | + | Ratio = V<sub>Pb</sub>/V<sub>tot</sub> =67% |
=== Plan 2=== | === Plan 2=== | ||
| Line 71: | Line 74: | ||
{| border="1" cellpadding="20" cellspacing="0" | {| border="1" cellpadding="20" cellspacing="0" | ||
|- | |- | ||
| − | | | + | |Measurements |
| + | |Descriptions | ||
|V<sub>H2O</sub> = V<sub>sp</sub> (<math>cm^3</math>) | |V<sub>H2O</sub> = V<sub>sp</sub> (<math>cm^3</math>) | ||
|V<sub>tot</sub> (<math>cm^3</math>) | |V<sub>tot</sub> (<math>cm^3</math>) | ||
| + | |Ratio | ||
|- | |- | ||
| − | |1 ||2600 || 6500 | + | |1 ||without shaking ||2600 || 6500 ||60% |
|- | |- | ||
| − | |2 | + | |2 ||without shaking ||840 ||2000 ||58% |
| + | |- | ||
| + | |2 ||with a lot of shaking ||210 ||510 ||64.7% | ||
|- | |- | ||
|} | |} | ||
| Line 84: | Line 91: | ||
Calculations | Calculations | ||
| − | + | Ratio = 1 - V<sub>sp</sub>/V<sub>tot</sub> | |
| + | |||
| + | == Conclusion== | ||
| − | + | We believe the plan 2 is more precise. The reason is in the plan 1 the scales we had are not precise and there are error propagation when use mass to calculate volume. However, in plan 2 we directly measured the volume. | |
| − | + | Experiment also shows that shaking will increase the packing ratio significantly. | |
| − | + | [http://wiki.iac.isu.edu/index.php/PhotoFission_with_Polarized_Photons_from_HRRL Go Back] | |
| − | |||
Latest revision as of 06:26, 5 February 2009
Plan 1
By Mass
1 - Empty box, m 1
2 - Box filled with Lead, m 2
3 - Box filled with Lead and H 2 0, m 3
4 - Box filled with H 2 0, m 4
m Pb = m 2 - m 1
V Pb = (m 2 - m 1) / ρ Pb
m H 2 0 = m 3 - m 2
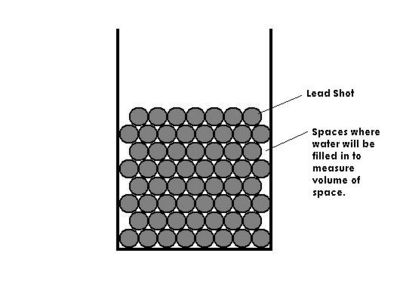
V H 2 0 = (m 3 - m 2) / ρ Pb = V SP (The space between the lead)
ratio = V Pb / V tot
Plan 2
By Volume
1 - V H 2 0 1 = V tot
2 - V H 2 0 2 = V SP
V Pb = V tot - V SP
R = V Pb / V tot = (V tot - V SP) / V tot => 1 - (V SP / V tot)
Experiment
Plan 1
We had 2 scales to measure the weight. Let's call them scale #1 and scale #2, measurement on them were called weight #1 and Weight respectively.
| Mesurements | Discriptions | masses | weight #1 (£) | weight #2 (£) | Weight AVE (£) | Weight AVE (g) |
| 1 | Box | m 1 | 3 | 4 | 3.5 | 1587.6 |
| 2 | Box+Pb | m 2 | 102 | 103 | 102.5 | 46493.2 |
| 3 | Box+Pb+O | m3 | 105 | 111 | 108 | 48988.0 |
| 4 | Box+O | m4 | 15 | 18 | 16.5 | 7484.3 |
Calculations
VPb = (m2 - m1)/ρPb=3959.2
VPb = (m4 - m1)/ρH2O=5896.8
Ratio = VPb/Vtot =67%
Plan 2
| Measurements | Descriptions | VH2O = Vsp () | Vtot () | Ratio |
| 1 | without shaking | 2600 | 6500 | 60% |
| 2 | without shaking | 840 | 2000 | 58% |
| 2 | with a lot of shaking | 210 | 510 | 64.7% |
Calculations
Ratio = 1 - Vsp/Vtot
Conclusion
We believe the plan 2 is more precise. The reason is in the plan 1 the scales we had are not precise and there are error propagation when use mass to calculate volume. However, in plan 2 we directly measured the volume.
Experiment also shows that shaking will increase the packing ratio significantly.