Difference between revisions of "Tony chapter 4"
| (5 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | [https://wiki.iac.isu.edu/index.php/Electronics_RS Go Back to All Lab Reports] | ||
| + | |||
=Semiconductor physics= | =Semiconductor physics= | ||
| Line 14: | Line 16: | ||
{| border="3" cellpadding="20" cellspacing="0" | {| border="3" cellpadding="20" cellspacing="0" | ||
| − | | [[File:TF_EIM_SimpleCubic.jpeg| | + | | [[File:TF_EIM_SimpleCubic.jpeg| 150 px]] || [[File:TF_EIM_BodyCentereCubic.jpeg| 150 px]] || [[File:TF_EIM_FaceCentereCubic.jpeg| 150 px]] |
|- | |- | ||
|(SC) Simple Cubic || (BCC) Body Centered Cubic|| (FCC) Face Centered Cubic | |(SC) Simple Cubic || (BCC) Body Centered Cubic|| (FCC) Face Centered Cubic | ||
| Line 29: | Line 31: | ||
| − | [[File:TF_EIM_Crystal_Cell_EnergyLevels.png| | + | [[File:TF_EIM_Crystal_Cell_EnergyLevels.png| 350 px]] |
;Notice | ;Notice | ||
| Line 169: | Line 171: | ||
| − | [[File:TF_EIM_SiliconLattice_np_junction.jpg| | + | [[File:TF_EIM_SiliconLattice_np_junction.jpg| 300 px]][[File:TF_EIM_p-n_junction.jpg| 300 px]] |
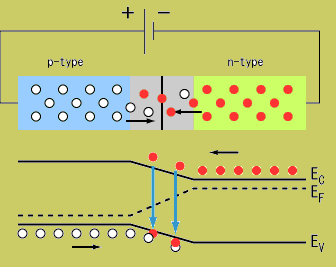
Note: The material still has a net charge of zero. The semi-conductor has excess FREE electrons on the n-side and excess HOLES on the p-side of the junction. Notice this looks like a charged up capacitor with a voltage difference to be in order for charge to move/conduct. | Note: The material still has a net charge of zero. The semi-conductor has excess FREE electrons on the n-side and excess HOLES on the p-side of the junction. Notice this looks like a charged up capacitor with a voltage difference to be in order for charge to move/conduct. | ||
| Line 179: | Line 181: | ||
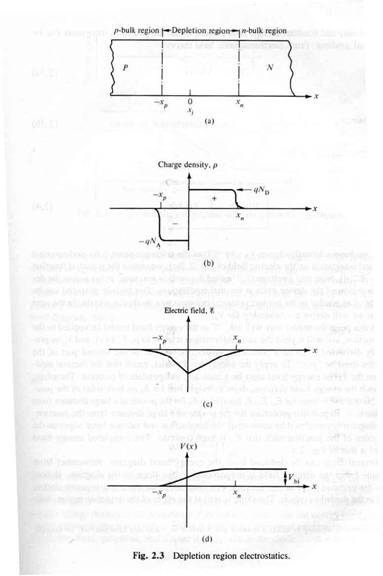
The "depletion" region is located at the junction and is a place which has been "depleted" of mobile charge carriers. The ionized acceptor and donor impurity atoms are fixed in the crystal lattice at the junction. | The "depletion" region is located at the junction and is a place which has been "depleted" of mobile charge carriers. The ionized acceptor and donor impurity atoms are fixed in the crystal lattice at the junction. | ||
| − | [[File:TF_EIM_PotBarrier_p-n_junct.jpg | | + | [[File:TF_EIM_PotBarrier_p-n_junct.jpg | 500 px]] |
| Line 203: | Line 205: | ||
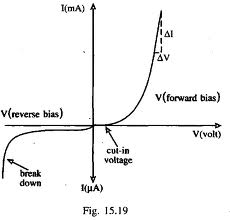
If you try and push current in the opposite direction you have an even higher barrier (step potential) to overcome than in the forward direction. There are only "holes" on the p-type side of the junction so electrons need to be "freed" by breaking covalent bonds on the p-type material side thereby creating 2 new charge carriers, one for the hole and one free to move to the n-type side. The bond breaking potential is higher than the potential needed to overcome the difference in Fermi energies. | If you try and push current in the opposite direction you have an even higher barrier (step potential) to overcome than in the forward direction. There are only "holes" on the p-type side of the junction so electrons need to be "freed" by breaking covalent bonds on the p-type material side thereby creating 2 new charge carriers, one for the hole and one free to move to the n-type side. The bond breaking potential is higher than the potential needed to overcome the difference in Fermi energies. | ||
| − | [[File:TF_EIM_Diode_V-vs-I_curve.jpg| | + | [[File:TF_EIM_Diode_V-vs-I_curve.jpg| 400 px]] |
[[Forest_Electronic_Instrumentation_and_Measurement]] | [[Forest_Electronic_Instrumentation_and_Measurement]] | ||
Latest revision as of 17:17, 16 February 2011
Semiconductor physics
There are 5 states of matter: Solid, liquid, gas, plasma (ionized gas) , and a Bose-Einstein condensate (quantum effects on a macroscopic scale).
Crystal Lattice
Semiconductor physics focuses on the solid state of matter in the form of crystals.
Crystals are formed when atoms are arranged in repeating structures called the crystal lattice. The recurrent structure which forms the crystal lattice is referred to a a cell.
Some popular cell names are
 |
 |
|
| (SC) Simple Cubic | (BCC) Body Centered Cubic | (FCC) Face Centered Cubic |
The above images are suppose to represent the distribution of electrons in the crystal lattice cell.
To understand this structure more let's consider the structure of a 1-D crystal of atoms
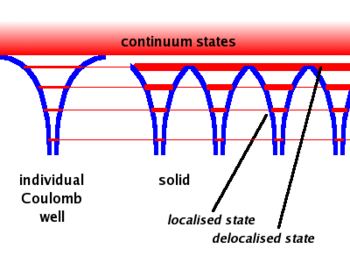
A single free atom will have electrons bound to the atom due to the coulomb force. The bound electrons are found in various energy levels.
- Notice
- When you add the atoms to form the lattice, the potential barrier is lowered. This allows the electrons in the higher (more valence) states to become shared among adjacent atoms.( Delocalized)
- Another effect is that the energy bands become wider. As the electrons in the higher energy states are shared between atoms forming a bond, the time any one electron spends bound to any one atom becomes smaller. SInce one finds that gets bigger for the higher state electrons (less bound) since their time \Delta t in a given bound state becomes less. The electron energy states become band like structures which get wider as you move to higher energy (less bound) electron states.
There are 14 different Bravais lattice configurations
| Crystal System | Lattice type |
| Cubic | Simple, Face Centered, Body Centered |
| Tetragonal | Simple, Body Centered |
| Orthorhombic | Simple, Face Centered, Body Centered, End Centered |
| Monoclinic | Simple, End Centered |
| Rhombohedral | Simple |
| Triclinic | Simple |
| Hexagonal | Simple |
Most Semiconductors are made from Silicon and GaAs.
Silicon is a Face Centered Cubic cell
Silicon is an insulator if in pure form with 4 weakly bound (valence) electrons.
If you replace silicon atoms in the lattice with atoms that have either 3 valance or 5 valence electrons (doping) your can create sites with either a deficient number of electrons (a missing bond) or extra electrons (complete bond with a free electron)
By Doping silicon you can create sites with extra electrons (n-type) or sites with a deficient number of electrons (vacancies or holes ) (P-type)
Fermi-Dirac Statistics
As seen above, the properties of a semi-conductor will be determined by the electron configuration in the crystal lattice. In particular, the distribution of electrons in the conduction band of the lattice since those are the electrons which can move in the crystal in response to an applied electric field (voltage) as produce observable effect.
Pauli Exclusion Principle
The Pauli exclusion principle states that no two electrons may occupy the same quantum state. This principle is seen by the quantum energy levels of electrons bound to atoms.
An important consequence of this principle is that, an crystal composed of atoms will filled energy bands is an insulator. You can only conduct electrons if there is an energy state available for the electron to populate. An electric field is established by applying a potential difference which pushes an electron up to a partially filled available energy state that is shared between the atoms in the lattice, thereby allowing the electron to "travel" to adjacent atoms.
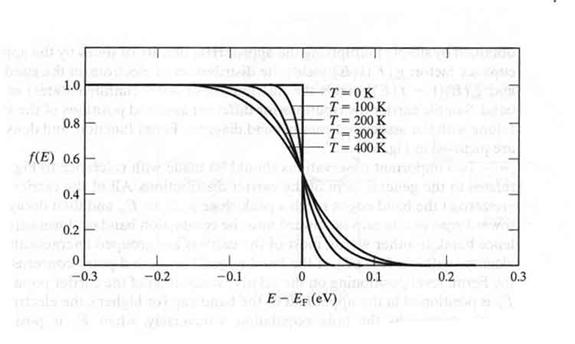
Fermi Function (F[E])
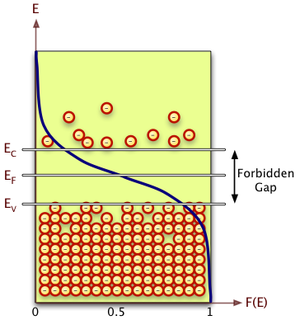
The energy levels in a crystal lattice may be grouped into a conduction band and a valence band that are separated by a forbidden energy region called the band gap.
the probability that a given energy level is occupied by an electron.
using Statistical Thermodynamic one can calculate the above Fermi function as
where
- Fermi energy of the given crystal lattice
- temperature of lattice in Kelvin
- Note
- At room temperature: kT = 1/40 eV
- (half of the energy needed to move from the valence to the conduction band
Electron Energy Distribution
The number of electrons per unit volume within the energy band is represented as
where
- density of states
When is in the conduction band
or
The total number of electrons in the conduction band is
Where
- Note: usually makes the exponential term more dominant than the term.
For the two common semi-conductors of Germainum and Silicon at room temperature we have
How much current can this be?
if we assume
cross-sectional area of the wire = electron drift velocity for Germanium when an electric field of 10V/cm is applied through the wire.
Then
A detectable current but not high enough for practical applications
The P-N junction Diode/rectifier
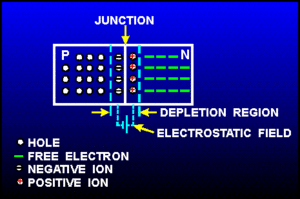
Returning back to the picture of a silicon lattice doped with Boron (3 instead of 4 valence e-; hole; acceptor atom) and Phosphorus ( 5 instead of 4 valence electrons; electron, donor atom).
Note: The material still has a net charge of zero. The semi-conductor has excess FREE electrons on the n-side and excess HOLES on the p-side of the junction. Notice this looks like a charged up capacitor with a voltage difference to be in order for charge to move/conduct.
AN electric field in the depletion region points from the n-type (donor) side to the p-type (acceptor) side. Electrons near the junction are pulled toward the n-type material and holes are pulled to the p-type material.
This electric field results in an effective voltage , where is the thickness of the depletion region.
The "depletion" region is located at the junction and is a place which has been "depleted" of mobile charge carriers. The ionized acceptor and donor impurity atoms are fixed in the crystal lattice at the junction.
A forward biasing of the above p-n junction means that you oppose the direct of the junction field. The positive side of the battery is put on the "p" side of the junction and the negative side on the "n" side of the junction. This reduces the junction barrier (squeezes the depletion region) making it easier for current to flow through the junction. The bias electric field is directed opposite to teh depletion electric field . When you reverse bias the p-n junction you are increasing the junction barrier making it harder for current to flow. At some point though, the junction will break down and you will force current to flow.
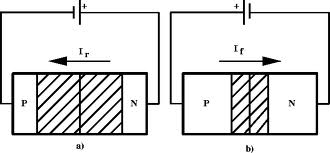
|
| Notice the depletion region expands for the reverse biased example on the left. The depletion region narrows when the p-n junction is in the forward biased orientation. |
If you try and push current in the opposite direction you have an even higher barrier (step potential) to overcome than in the forward direction. There are only "holes" on the p-type side of the junction so electrons need to be "freed" by breaking covalent bonds on the p-type material side thereby creating 2 new charge carriers, one for the hole and one free to move to the n-type side. The bond breaking potential is higher than the potential needed to overcome the difference in Fermi energies.