ProposalDeepBlueMarine 2013
Project Summary
We propose to measure the concentrations of several nuclei using photon activation analysis (PAA) and determine if there is a correlation between several coin samples and candidate mines that may have been the source for the silver in the coin. Based on previous work (insert NAA reference) and our preliminary results, we intend to focus on Gold, Irridum, and Strontium nuclei. PAA has been able to measure the content of nuclei in a sample to the level of parts-per-million (PPM). PAA has successfully performed elemental and matching analyses for a wide variety of samples including museum artifacts [1], fossils [2], forensic samples [3], agricultural samples [4], and various environmental samples [5-6]. PAA is an ideal tool for performing the above measurements due to its ability to accurately measure PPM level concentrations without destroying the sample as compared to other methods of detecting base elements [7 - 9].
A Description of PAA
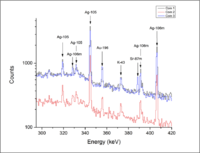
Photon Activation Analysis is a non-destructive technique used to measure the concentration of nuclei in materials. A sample is irradiated using bremsstrahlung photons at energies that are sufficient to excite the material's nuclei. After the irradiation, the sample is placed in a photon spectrometer to quantify the energy of the photons that are emitted when the nuclei within the material de-excite. The photon energy emitted by this de-excitation can be used to identify potential nuclear species. Measuring the decay time (half life) of specific photon energies can allow a determination of a unique nuclear species. By comparing the number of photons being emitted at the characteristic energy to a known standard it is possible to quantify the concentration of nuclei in the sample. Below are several examples that used this technique.
Previous results
PAA for Forensics
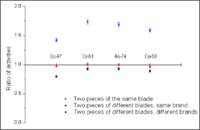
One of our latest projects performed a forensic analysis of several evidence samples from difference knives. We were investigating six samples: Sample 1 and Sample 2 were pieces of two identical knives of the same brand. Sample 3 and Sample 4 were pieces of a single knife. Sample 5 and Sample 6 were pieces of two knives from different manufacturers. All the samples had masses ranging from 2.5 to 4 grams and had approximately the same (but not identical) surface area. They were irradiated for 2 hours using a 30 MeV, 2 kW electron beam. Each samples's activity was measured after the irradiation using a high purity germanium detector. Several gamma emission lines, corresponding to Cr, Mo, Ni, Mn, Ti, Co, Sb, and As target nuclides, were easily identified. Figure 3 shows a comparison of the different element concentrations for the samples that were taken from one knife blade (red), from different blades of the same brand (black), and from different brands (blue). It’s clear that pieces of a particular knife blade can easily be differentiated. PAA can clearly distinguish between different batches of same-brand knives, and therefore can be an excellent evidence match analysis tool.
shall we include a statistical comparison? i.e.: several elements can distinguish between the different brand knives with a convidence level of (90%). There is also an indication that different knives from the same brand can be distinguished provided a PAA fingerprint for a knife.
Provinence searches using PAA
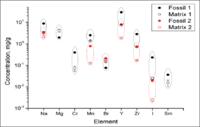
Another recent project performed a match analysis between two fossils and two soil matrices. We simultaneously irradiated the four samples collected from two separate locations in South Dakota using the bremsstrahlung photons generated by 20 MeV electron accelerator. After 5 hours of irradiation, we measured the energy spectrum of photons and compared the concentrations of different elements in the fossils and in the matrices (Figure 4). One striking result is Mg, Cr, and Sm were not detectable in Fossil 2 but were found in Fossil 1. Fossil 1 and soil Matrix 1 have detectable concentrations of Mg, Cr, and Sm that were not observable in Fossil 2 and Matrix 2, suggesting that a strong correlation may exist. When comparing all elements there appears to be a strong correlation between Fossil 1 and soil Matrix 1 as well as a correlation between Fossil 2 and soil Matrix 2. In particular, Fossil 1 and soil Matrix 1 have a higher concentration of Na, Mn, Y, Zr, and I. Fossil 2 and soil Matrix 2, on the other hand, has higher concentration of Br. Thus we were able to show that there is a strong correlation between the elemental composition of the fossil and the elemental composition of the corresponding matrix.
Preliminary results
In March of 2013, three silver coins were provided by Deep Blue Marine to measure the spectrum of photon energies emitted by irradiated coins. These measurements were the first step towards using PAA to perform a multivariate statistical analysis that can test the origin of the coins. As in the provenance study of the fossils above, there is an element present in one sample but missing in others. This element was metastable state of Sr-87. The difference is our fist indication that a correlation may be determine between the coin sample and the mine which produced the ore for the core. We propose to measure several mine samples to determine if this correlation does exist.
Proposed Work
We are proposing to perform the following tasks:
1)Experiment preparation: Estimation of photon flux and prediction of activities for radiation safety
After the number of samples, their shape ,and mass are known, a Monte-Carlo simulation will be performed to estimate the photon flux through the samples and choose an optimum irradiation conditions, such as:
- positioning of the samples - time of irradiation - electron beam parameters, such as electron energy and average current
The simulations will be performed using a special radiation transport software, MCNP, at the Idaho Accelerator Center computer cluster (12 nodes, 52 cores, 64GB of aggregate memory, and 108GHz of aggregate CPU power).
2) Sample irradiation and gamma-spectrometry
Once the irradiation conditions are established, samples (artifacts and ores) will be irradiated for a short time (10-30 minutes) for two purposes: do qualitative analysis of elemental composition and compare the predictions of photon flux and activities. After making sure the activities are within expectations and compliant with radiation safety protocols, the samples and reference material, can be irradiated for 8-10 hours to maximize PAA sensitivity of the technique and minimize the detection limit. After irradiation, the samples’ elemental composition will be measured using a high purity germanium detector. All the spectra will be stored.
3) Data analysis
Gamma spectra will be analyzed. Peaks in the spectra will be identified and the concentration of observable elements of interest will be calculated from the produced radioactivity.
4) Final report
Measured activities will be described in details. We will provide the description of the experiment, raw spectroscopic dat,a and a table of calculated concentrations of different elements. Error analysis will be performed and uncertainties will be included. The work undertaken as part of this proposal will be completed 60 days after an award is received.
Budget
Justification
The budget below quantifies a cost breakdown for irradiating a set of five coins for 10 hours and a set of 3 ore samples for 10 hours. The PI will be responsible for managing the project, performing irradiations, measuring spectra, and delivering a report. It is estimated that the PI will spend two weeks performing the tasks needed to meet the project deliverables. A PAA consultant, with an established record of PAA analysis, will be hired to perform the spectra analysis and will be responsible for quantifying the concentration of the indicated isotopes. It is expected that the analysis consultant will spend approximately one week on the above tasks. A student
| Budget = $19,746.40 | |
| Category | Cost |
| PI | 3692 |
| Consultant | 4000 |
| Student | 1760 |
| Beam time | 5000 |
| Travel | 1500 |
| Shipping | 200 |
| Consumables | 100 |
| Fringe | 931.96 |
| Indirects | 2562.44 |
Bibliography
[1] C. Segebade, “Investigation of a medieval sword using photon activation analysis”, Journal of Radioanalytical and Nuclear Chemistry, 169, 1, pp 27-37, 1993 [2] V. Starovoitova, P. Cole, C. Segebade, “Multi-element photon activation analysis of fossil samples”, 2013 (in preparation)
[3] V. Starovoitova, P. Cole, C. Segebade, “Forensic evidence sample matching using photon activation analysis”, 2013 (in preparation)
[4] Z. J. Sun, D. P. Wells, C. Segebade, H. Maschner, B. Benson, “A provenance study of coffee by photon activation analysis”, Journal of Radioanalytical and Nuclear Chemistry, in print, 2012
[5] Mamtimin M, Cole P, Segebade C. “Photon activation analysis of dust particles for environmental research and applications using the 44 MeV electron LINAC at the Idaho accelerator center”, AccApp ‘11—tenth international topical meeting on nuclear applications of accelerators, Knoxville, TN [6] Thümmel HW, Segebade C, Hirsch K., “Optimization of irradiation conditions for photon activation analysis of biological and environmental samples using a high power electron accelerator”, Biol Trace Elem Res., 43-45:141-9, 1994 [7] Segebade C, Weise HP, Lutz GJ (1987) Photon activation analysis. W. de Gruyter, Berlin
[8] Segebade, C., A Schmitt, B.F., “Analysis of high-purity material—A comparison of photon activation analysis with other instrumental methods”, Journal of Radioanalytical and Nuclear Chemistry. 113, 1, pp 61-76, 1983
[9] Neider, R., Dudzus, Th., Fusban, H.U., Jost, P., Reimers, P., Schmitt, B.F., Segebade, Chr., Wandelburg, K., Weise, H.P., “Comparison of photon activation analysis with other modern analytical methods as tools for the solution of actual analytical problems”, Journal of Radioanalytical and Nuclear Chemistry, 39, 1-2, pp 397-408, 1977