Difference between revisions of "ForestSolarCells"
| (17 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | = What is a solar cell= | + | = What is a solar cell ?= |
| − | Solar cells are designed to convert light into | + | Solar cells are designed to convert light into electricity. They do this without the use of either chemical reactions or moving parts. Modern solar cells are based on semiconductors, a material whose electrical properties lie between conductors and insulators. In a conductor, electric current can flow freely. Electric current can't flow freely in an insulator. Light hitting a semiconductor solar cell pushes electrons out of the semiconductor making an electric current we can use. The first material known to behave as a semiconductor was Cuprous Oxide <math>(Cu_2O)</math> and was used as early as 1924. Descriptions of how to make a solar cell using Cupric Oxide suggest putting the copper sheets in a salt water solution. I wanted to know if the amount of salt used changed the amount of current you could get. |
| − | Does salt change the amount of current? | + | Does salt change the amount of current? |
| − | + | ||
| − | + | =Materials I needed= | |
1. A half square foot sheet of copper flashing. | 1. A half square foot sheet of copper flashing. | ||
| Line 33: | Line 33: | ||
I learned that the amount of salt doesn't change the current. | I learned that the amount of salt doesn't change the current. | ||
| − | |||
=Making Cuprous Oxide = | =Making Cuprous Oxide = | ||
| − | Cuprous oxide <math>(Cu_2O)</math> can be easily made by heating thin sheets of copper. Heating the copper causes the formation of both cuprous oxide and cupric oxide <math>(CuO)</math>. Cupric oxide has one Copper atom attached to one Oxygen atom and is not what we want. Underneath the dark black cupric oxide is cuprous oxide which has two Copper atoms attached to one Oxygen atom.. Cuprous oxide looks reddish. I first tried to make cuprous oxide using the top burner on an electric stove. As the copper sheet heated | + | Cuprous oxide <math>(Cu_2O)</math> can be easily made by heating thin sheets of copper. Heating the copper causes the formation of both cuprous oxide and cupric oxide <math>(CuO)</math>. Cupric oxide has one Copper atom attached to one Oxygen atom and is not what we want. Underneath the dark black cupric oxide is cuprous oxide which has two Copper atoms attached to one Oxygen atom.. Cuprous oxide looks reddish. I first tried to make cuprous oxide using the top burner on an electric stove. As the copper sheet heated it changed color until it was all black on the surface. As it cooled off the black cupric oxide fell off revealing some reddish cupric oxide underneath. I did not see a lot of the reddish cuprous oxide formed. I decided to use an oven to heat the copper sheet more evenly |
and at a high temperature (500 celsius). This time I saw a lot of the reddish cuprous oxide. | and at a high temperature (500 celsius). This time I saw a lot of the reddish cuprous oxide. | ||
| Line 51: | Line 50: | ||
[[Image:Copper_cuprousocideRed_3-1-08.jpg | 200 px]] | [[Image:Copper_cuprousocideRed_3-1-08.jpg | 200 px]] | ||
| − | Aparatus | + | =Aparatus= |
| + | |||
| + | A 2 liter plastic container, an ammeter, wire leads, and water were used to measure the current produced by the cupric oxide solar cell. The plastic container was filled with 1.8 Liters of water. A copper sheet, which was not heated, was placed on one side of the container and the copper sheet with cupric oxide was placed on the opposite side. The positive lead of the ammeter was connected to the cupric oxide sheet and the ground of the ammeter was connected to the plain copper sheet. The current was measured by the ammeter under direct sunlight and sunlight blocked by a curtain. Several measurements were made using different amount of salt ranging from 0 to 10 teaspoons. | ||
[[Image:SolarCellApparatus_3-8-08.jpg | 200 px]] | [[Image:SolarCellApparatus_3-8-08.jpg | 200 px]] | ||
| + | =Current measurements= | ||
| − | + | The pictures below show the deflection of the ammeter when the sun was blocked and then unblocked. The ammeter measured 8 <math>\mu</math>A with little sunlight and 16 <math>\mu</math>A with sunlight. | |
[[Image:Ammeter_NoSunlight.jpg | 200 px]] | [[Image:Ammeter_NoSunlight.jpg | 200 px]] | ||
[[Image:Ammeter_Sunlight.jpg | 200 px]] | [[Image:Ammeter_Sunlight.jpg | 200 px]] | ||
| + | |||
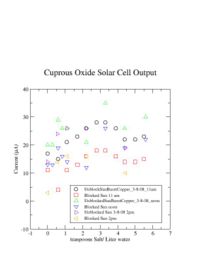
| + | The current was measured 4 different times and for several amount of salt. The graph below shows 3 measurements of the current both with the sunlight blocked and unblocked at three different times during a partly cloudy day on March 8, 2008. The current for each case appeared to change a lot. | ||
[[Image:RawData_2-8-08.jpg | 200 px]] | [[Image:RawData_2-8-08.jpg | 200 px]] | ||
| + | |||
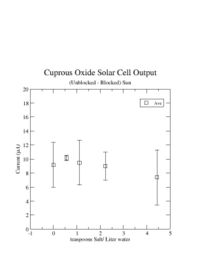
| + | The graph below shows the average current measured from sunlight after subtracting out the current measured with the sunlight blocked. The subtraction of the blocked sunlight current resulted in a current measurement which was repeatable. The vertical lines on each point represent the fluctuation in the measured current. The points show a clear non-zero current is being made by the solar cell. Other people have seen values between 6 and 50 <math>\mu</math>A (see http://scitoys.com/scitoys/scitoys/echem/echem2.html). It is also difficult to see any dependence on the amount of salt put into the water. | ||
[[Image:DiffData_2-8-08.jpg | 200 px]] | [[Image:DiffData_2-8-08.jpg | 200 px]] | ||
| + | |||
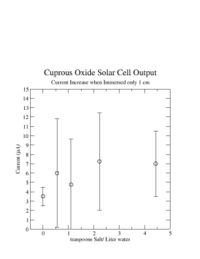
| + | I also noticed during this experiment that the current would increase if I lifted the cuprous oxide sheet out of the water until only about 1 cm of it was still in the water. The graph below shows that the current increase by about 4 <math>\mu</math>A if I moved the cuprous oxide sheet such that only 1 cm was in the water. I did not see the same thing happen if I moved the copper sheet out. | ||
[[Image:ImersedIn1cmData_2-8-08.jpg | 200 px]] | [[Image:ImersedIn1cmData_2-8-08.jpg | 200 px]] | ||
| + | |||
| + | =Conclusions= | ||
| + | |||
| + | #I was unable to see the current change if I added 0 to 10 teaspoon of salt to 1.8 L of water. | ||
| + | #I was the current increase if I only put the cuprous oxide sheet 1 cm into the water. | ||
| + | #I was able to make more cuprous oxide using an oven than a stove top | ||
Latest revision as of 22:35, 9 March 2008
What is a solar cell ?
Solar cells are designed to convert light into electricity. They do this without the use of either chemical reactions or moving parts. Modern solar cells are based on semiconductors, a material whose electrical properties lie between conductors and insulators. In a conductor, electric current can flow freely. Electric current can't flow freely in an insulator. Light hitting a semiconductor solar cell pushes electrons out of the semiconductor making an electric current we can use. The first material known to behave as a semiconductor was Cuprous Oxide and was used as early as 1924. Descriptions of how to make a solar cell using Cupric Oxide suggest putting the copper sheets in a salt water solution. I wanted to know if the amount of salt used changed the amount of current you could get.
Does salt change the amount of current?
Materials I needed
1. A half square foot sheet of copper flashing.
2. Two alligator clip leads.
3. A sensitive micro-ammeter that can read currents between 10 and 50 microamperes.
4. An electric stove or oven.
5. A large clear plastic bottle with the top cut off. I used a 2 liter spring water bottle. A large mouth glass jar will also work.
6. Table salt.
7. Tap water.
8. Sand paper or a wire brush.
9. A Paper cutter for cutting the copper sheet.
I was unable to observe a dependence of the solar cell output current on the amount of salt I added.
The standard setup for my project is a plastic bottle that contains hot or cold water, salt, and 2 sheets of copper. One of the copper sheets was baked at a high temperature to convert the copper into cuprous oxide. The alligator clips are used to attached to the micro ammeter to the two copper sheets. The micro ammeter measures how much current flows between the two copper sheets.
I learned that the amount of salt doesn't change the current.
Making Cuprous Oxide
Cuprous oxide can be easily made by heating thin sheets of copper. Heating the copper causes the formation of both cuprous oxide and cupric oxide . Cupric oxide has one Copper atom attached to one Oxygen atom and is not what we want. Underneath the dark black cupric oxide is cuprous oxide which has two Copper atoms attached to one Oxygen atom.. Cuprous oxide looks reddish. I first tried to make cuprous oxide using the top burner on an electric stove. As the copper sheet heated it changed color until it was all black on the surface. As it cooled off the black cupric oxide fell off revealing some reddish cupric oxide underneath. I did not see a lot of the reddish cuprous oxide formed. I decided to use an oven to heat the copper sheet more evenly and at a high temperature (500 celsius). This time I saw a lot of the reddish cuprous oxide.
The picture above shows the difference between the two burnt copper sheets. The sheet on the left was baked at 500 celsius and the one on the right was cooked on a stove top. You can see that the baked copper sheet has more of the red cuprous oxide visible while the one burnt on the stove top has the red cuprous oxide located mostly in the lower half of the sheet.
A 6" x 6" copper flashing was baked in a 500 degree celsius oven for 20 Minutes.
Aparatus
A 2 liter plastic container, an ammeter, wire leads, and water were used to measure the current produced by the cupric oxide solar cell. The plastic container was filled with 1.8 Liters of water. A copper sheet, which was not heated, was placed on one side of the container and the copper sheet with cupric oxide was placed on the opposite side. The positive lead of the ammeter was connected to the cupric oxide sheet and the ground of the ammeter was connected to the plain copper sheet. The current was measured by the ammeter under direct sunlight and sunlight blocked by a curtain. Several measurements were made using different amount of salt ranging from 0 to 10 teaspoons.
Current measurements
The pictures below show the deflection of the ammeter when the sun was blocked and then unblocked. The ammeter measured 8 A with little sunlight and 16 A with sunlight.
The current was measured 4 different times and for several amount of salt. The graph below shows 3 measurements of the current both with the sunlight blocked and unblocked at three different times during a partly cloudy day on March 8, 2008. The current for each case appeared to change a lot.
The graph below shows the average current measured from sunlight after subtracting out the current measured with the sunlight blocked. The subtraction of the blocked sunlight current resulted in a current measurement which was repeatable. The vertical lines on each point represent the fluctuation in the measured current. The points show a clear non-zero current is being made by the solar cell. Other people have seen values between 6 and 50 A (see http://scitoys.com/scitoys/scitoys/echem/echem2.html). It is also difficult to see any dependence on the amount of salt put into the water.
I also noticed during this experiment that the current would increase if I lifted the cuprous oxide sheet out of the water until only about 1 cm of it was still in the water. The graph below shows that the current increase by about 4 A if I moved the cuprous oxide sheet such that only 1 cm was in the water. I did not see the same thing happen if I moved the copper sheet out.
Conclusions
- I was unable to see the current change if I added 0 to 10 teaspoon of salt to 1.8 L of water.
- I was the current increase if I only put the cuprous oxide sheet 1 cm into the water.
- I was able to make more cuprous oxide using an oven than a stove top