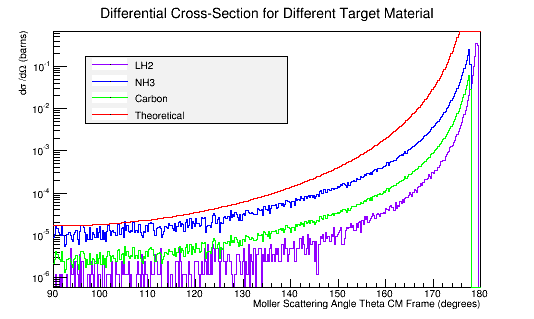
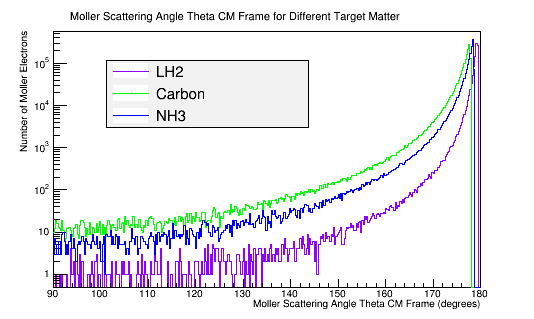
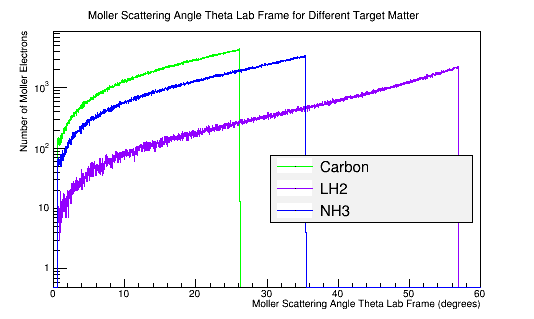
Calculating the differential cross-sections for the different materials, and placing them as well as the theoretical differential cross-section into a plot:
Jump to navigation
Jump to search
Comparing this to the theoretical differential cross section
As shown above , we find that the differential cross section scale is
Converting the number of electrons to barns,
where ρtarget is the density of the target material, ltarget is the length of the target, and iscattered is the number of incident particles scattered.
For LH2:
For Carbon:
For Ammonia:
Combing plots in Root:
new TBrowser();
TH1F *LH2=new TH1F("LH2","LH2",360,90,180);
LH2->Add(MollerThetaCM,1.19e-6);
LH2->Draw();
TH1F *C12=new TH1F("C12","C12",360,90,180);
C12->Add(MollerThetaCM,2.21e-7);
C12->Draw();
TH1F *NH3=new TH1F("NH3","NH3",360,90,180);
NH3->Add(MollerThetaCM,8.87e-7);
NH3->Draw();
LH2->Draw("same");
C12->Draw("same");
Theory->Draw("same");
| Material | Molar Mass (g/mole) |
|---|---|
| NH3 | 17 |
| C | 12 |
| LH2 | 2 |
| Material | Number of Electrons |
|---|---|
| NH3 | 10 |
| C | 6 |
| LH2 | 2 |
The greater the molar mass, the smaller the solid angle into which the Moller electron will scatter.
Comparing this with a plot of the Moller scattering angle theta,
Density of target material
C=2.26 g/cm3
NH3=.86g/cm3
LH2=.07g/cm3
The greater the density, the smaller the solid angle into which the Moller electron will scatter.