Difference between revisions of "Calculating the differential cross-sections for the different materials, and placing them as well as the theoretical differential cross-section into a plot:"
| Line 119: | Line 119: | ||
| 0.07 | | 0.07 | ||
|} | |} | ||
| + | |||
| + | |||
| + | |||
Revision as of 17:20, 15 April 2016
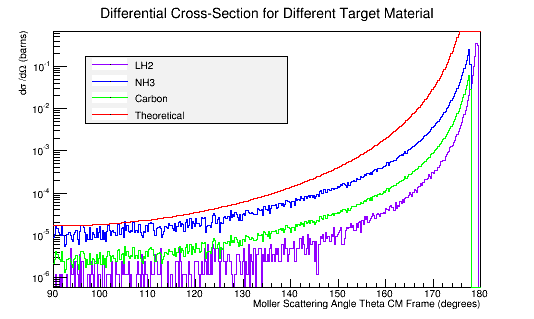
Comparing this to the theoretical differential cross section
As shown above , we find that the differential cross section scale is
Converting the number of electrons to barns,
where ρtarget is the density of the target material, ltarget is the length of the target, and iscattered is the number of incident particles scattered.
For LH2:
For Carbon:
For Ammonia:
Combing plots in Root:
new TBrowser();
TH1F *LH2=new TH1F("LH2","LH2",360,90,180);
LH2->Add(MollerThetaCM,1.19e-6);
LH2->Draw();
TH1F *C12=new TH1F("C12","C12",360,90,180);
C12->Add(MollerThetaCM,2.21e-7);
C12->Draw();
TH1F *NH3=new TH1F("NH3","NH3",360,90,180);
NH3->Add(MollerThetaCM,8.87e-7);
NH3->Draw();
LH2->Draw("same");
C12->Draw("same");
Theory->Draw("same");
| Material | Molar Mass (g/mole) |
|---|---|
| NH3 | 17 |
| C | 12 |
| LH2 | 2 |
| Material | Number of Electrons |
|---|---|
| NH3 | 10 |
| C | 6 |
| LH2 | 2 |
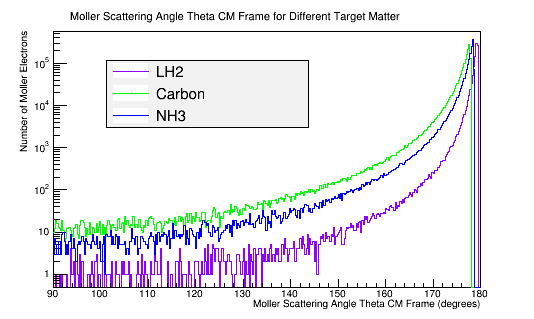
The molar mass is proportional to the number of electrons in the target material. The theoretical Moller differential cross-section is an expression for a single scattering electron. NH3, as a molecule, is composed of 10 electrons that all have an equal momentum and probability of scattering with the incident electron in the CM frame.
Comparing this with a plot of the Moller scattering angle theta,
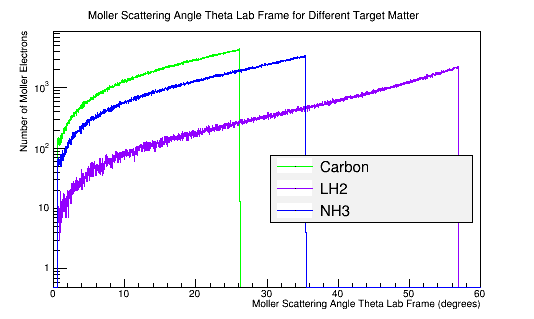
| Material | Density (g/cm3) |
|---|---|
| C | 2.26 |
| NH3 | 0.86 |
| LH2 | 0.07 |
In the lab frame, the incident electron, and the transfer of its momentum, depends on the stationary target material density. The more dense material requires more energy to break the bonds