Difference between revisions of "TF Antimony"
| Line 13: | Line 13: | ||
1.) How low of an Se concentration are they able to detect in the columns? | 1.) How low of an Se concentration are they able to detect in the columns? | ||
| − | + | Answer: nanograms | |
| Line 20: | Line 20: | ||
==Year 2==Based on the results from year 1, acquire natural Tellurium samples of masses from 1 g to 25 grams to confirm scalability of the irradiation method. The separation system needed 36 hours to separate Te-119m from a 25 g mass of natural Sb that was irradiated with protons. A large portion of the time was needed to dissolve the 25 g mass of Se. While the mg masses sent to ILANL in year one can go through the separation process in a day, the larger masses will require more time for dissolving and possibly waiting for sample activity to decrease. Instead consuming 24 hours shipping the sample to LANL, we will install a separation system at the IAC substantially shortening the delay from stopping the irradiation to processing. The dominant delay becomes how long we need to wait for the activity of the large mass samples to diminish so they may be handled safely. Do we need a hot cell at the IAC? | ==Year 2==Based on the results from year 1, acquire natural Tellurium samples of masses from 1 g to 25 grams to confirm scalability of the irradiation method. The separation system needed 36 hours to separate Te-119m from a 25 g mass of natural Sb that was irradiated with protons. A large portion of the time was needed to dissolve the 25 g mass of Se. While the mg masses sent to ILANL in year one can go through the separation process in a day, the larger masses will require more time for dissolving and possibly waiting for sample activity to decrease. Instead consuming 24 hours shipping the sample to LANL, we will install a separation system at the IAC substantially shortening the delay from stopping the irradiation to processing. The dominant delay becomes how long we need to wait for the activity of the large mass samples to diminish so they may be handled safely. Do we need a hot cell at the IAC? | ||
| − | ==Year 3== Optimize the Se-119 production method using Natural Te and in house separation. Determine the performance of continuous production over a two month period. Irradiate material once or twice a week to reload the separation system and build up an Isotope sample of Te-119m. Measure the output of Se-119 over the period of a month. | + | ==Year 3== Optimize the Se-119 production method using Natural Te and in house separation. Determine the performance of continuous production over a two month period. Irradiate material once or twice a week to reload the separation system and build up an Isotope sample of Te-119m. Measure the output of Se-119 over the period of a month. |
| − | |||
| − | |||
| − | |||
=Tellurium(Te) to Antimony(Sb)= | =Tellurium(Te) to Antimony(Sb)= | ||
Revision as of 09:31, 22 November 2023
Antiomony(Sb)
Proposal
We propose to develop an alternative stream for producing Sb-119 as an auger electron source for radiotherapy by irradiating Te-120 with bremstrahlung photons from a 40 MeV electron linac. Recently, antimony activities of a curie were produced using 25 gram antimony targets irradiate with protons at LANL. The chemical separation of tellurium and antimony after 36 hours was demonstrated indicating the potential for mass production of Sb-119 for radiotherapy.
Research Plan
Questions:
1.) How low of an Se concentration are they able to detect in the columns?
Answer: nanograms
===Year 1:== Procure 6, 1 mg powder samples of Te-120 ($24k). Irradiate one sample and measure the gamma spectrum for 511's as well as try to identify contaminants. Irradiate the previous sample and a new sample sending the new sample to LANL for separation and measure the gamma spectra of the other. Goal will be to measure a correlation between the observed gamma spectrum (511's from the Te-119->Se-119 decay) and the amount of Se-119 that is separated. Irradiate another sample to improve Se production based on the separation results of the first sample (may need to adjust irradiation times, target mass, target geometry,...). Try to revert the aqueous solution of Te that was separated back to powder form for a repeated irradiation.
==Year 2==Based on the results from year 1, acquire natural Tellurium samples of masses from 1 g to 25 grams to confirm scalability of the irradiation method. The separation system needed 36 hours to separate Te-119m from a 25 g mass of natural Sb that was irradiated with protons. A large portion of the time was needed to dissolve the 25 g mass of Se. While the mg masses sent to ILANL in year one can go through the separation process in a day, the larger masses will require more time for dissolving and possibly waiting for sample activity to decrease. Instead consuming 24 hours shipping the sample to LANL, we will install a separation system at the IAC substantially shortening the delay from stopping the irradiation to processing. The dominant delay becomes how long we need to wait for the activity of the large mass samples to diminish so they may be handled safely. Do we need a hot cell at the IAC?
==Year 3== Optimize the Se-119 production method using Natural Te and in house separation. Determine the performance of continuous production over a two month period. Irradiate material once or twice a week to reload the separation system and build up an Isotope sample of Te-119m. Measure the output of Se-119 over the period of a month.
Tellurium(Te) to Antimony(Sb)
A pure Tellurium foils is immersed in a bremsstrahlung beam to eject a proton from Te-120 leaving the Antimony isotope Se-119. Se-119 has a 38 hour half life. Se-119 emits auger electrons ( electrons emitted when a high level electron moves down to fill a vacancy). X-rays may also be produced when an electron moves down to fill a vacancy, the dominant X-rays emitted have energies of about 25 keV. Sb-119 decays to Sn-119 when a proton converts to a neutron plus a positron. The resulting Sn-119 is always in an excited state and decays in 18 ns to its ground state giving off a photon of energy 24 keV.
\gamma + Te-120 -> Sb-119 + p
Yield Problem: Te-120 is only 0.1% of the natural abundance. Highest natural abundance is Te-126 at 19%, then Te-125 at 7%, Te-124 at 5%, Te-122 at 2.5%, Te-123 at 1%.
A powder that is 50% Te-120 by volume can be procured for < $5k
One can also go to Te-119 from Te-120. Te-119 has a 16 hour half life (4 day half life if left in metastable state) before it beta+ decays to Sb-119. The emitted positron has an energy of about 285 keV (this produces a 511 keV positron annihilation signature). The dominant gamma line is at 644 keV. The metastable state is 261 keV above the ground state and also beta+ decays.
Melting point of foils is 450 C
Sb-119 decays by emitting K-edge and conversion electrons, collectively called Auger electrons.
Sb-117 is a PET analog => in 2 hrs Sb-117 decays emitting a positron that will annihilate and produce two 511 keV photons for a PET imager to detect.
Theradiagnostic is an therapy with companion atoms. One atom serves as the radiological therapy and the other atom emits radiation that is detectable to a diagnostic device. For example Sb-119 emits low energy auger electrons to kill cancer cells while Sb-117 beta decays emitting a positron that annihilates and emits two 511 photons that are detectable by a PET scanner. The half life of Sb-117 is less than 3 hours and the half life of Sb-119 is 38 hours.
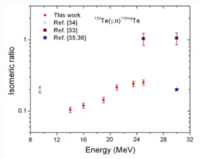
X-sect
[1] "The isomeric ratios in photonuclear reactions of natural tellurium induced by bremsstrahlungs with endpoint energies in the giant dipole resonance region", Tran Duc Thiep, Truong Thi An, Phan Viet Cuong, Nguyen Tuan Khai, Nguyen The Vinh, A. G. Belov & O. D. Maslov , Journal of Radioanalytical and Nuclear Chemistry volume 289, pages 637–645(2011)
We have to note that in this work the isomeric ratio was determined as the ratio of the yield of the high spin Y(hs) to that of the low spin states Y(ls) i.e. 𝐼𝑅 = Y(hs)/Y(ls).
Competition
Cyclotrons
Sn-119 +p -> Sb-119 + n
From Large Scale Production of 119mTe and 119Sb for Radiopharmaceutical Applications
Separating Sn-119 from Sb-119 reqires more than a half life of Sb-119
as a result you get 0.001 Ci in 10 days
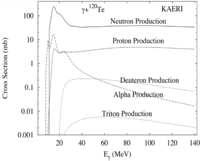
Proton irradiation
From Large Scale Production of 119mTe and 119Sb for Radiopharmaceutical Applications
Sb-121 + p -> Te-119m + 3n -> Sb-119
Sb-123 + p -> Te-119m + 5n -> Sb-119
4 day halflife for Te-119m to decay to Sb-119
0.001 Ci in 45 days
Separating Sb from Te
use anion-exchange chromatographic
Radiochemical separation of antimony and tellurium in isotope production and in radionuclide generators, D. Downs & D. A. Miller, Journal of Radioanalytical and Nuclear Chemistry volume 262, Article number: 241 (2004)
A LANL group (Michael fassbender)is working on creating a generator that separates tellurium from antimony. File:Bennet Se-Sb Sparation 2019.pdf
References
Large Scale Production of 119mTe and 119Sb for Radiopharmaceutical Applications ,Kevin T. Bennett, Sharon E. Bone, Andrew C. Akin, Eva R. Birnbaum, Anastasia V. Blake, Mark Brugh, Scott R. Daly, Jonathan W. Engle, Michael E. Fassbender, Maryline G. Ferrier, Stosh A. Kozimor*, Laura M. Lilley, Christopher A. Martinez, Veronika Mocko, Francois M. Nortier, Benjamin W. Stein, Sara L. Thiemann, and Christiaan Vermeulen, Cite this: ACS Cent. Sci. 2019, 5, 3, 494–505 Publication Date:February 25, 2019 https://doi.org/10.1021/acscentsci.8b00869
Accelerator based Production of Auger-Electron-emitting Isotopes for Radionuclide Therapy Helge ThisgaardR, PhD-theis, pg 22; Thesis may have led to this publication Thisgaard H.; Jensen M. Production of the Auger emitter 119Sb for targeted radionuclide therapy using a small PET-cyclotron. Appl. Radiat. Isot. 2009, 67, 34–38.
The Paradox of Using Radionuclides To Treat Disease, Thomas E. Albrecht-Schmitt, ACS Cent Sci. 2019 Mar 27; 5(3): 383–385
Anion exchange separation of tin, antimony and tellurium, GilbertW. SmithS.A.Reynolds , Analytica Chimica Acta
Volume 12, 1955, Pages 151-153