Difference between revisions of "Monte Carlo Binary Collision Approximation"
Jump to navigation
Jump to search
| Line 1: | Line 1: | ||
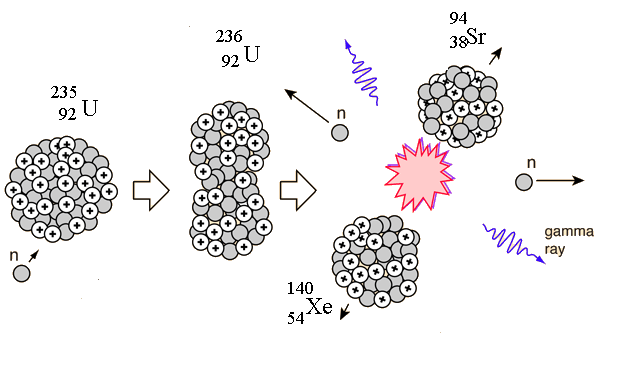
| − | When uranium-235 undergoes fission, the average of the fragment mass is about 118, but it is more probable that the pair will have an unequal distribution in mass. A common pair of fragments from uranium-235 fission is xenon and strontium: | + | When uranium-235 undergoes fission, the average of the fragment mass is about 118, but it is more probable that the pair will have an unequal distribution in mass. A common pair of fragments from uranium-235 fission is xenon and strontium as shown in the reaction: |
| Line 6: | Line 6: | ||
<center>[[File:U235_fission_Xe_Sr.png|"thumb"|"border"|"center"|"middle"|"upright"|||page=Page||Figure 1: Typical Uranium 235 fission fragments Xenon and Strontium. ]]</center> | <center>[[File:U235_fission_Xe_Sr.png|"thumb"|"border"|"center"|"middle"|"upright"|||page=Page||Figure 1: Typical Uranium 235 fission fragments Xenon and Strontium. ]]</center> | ||
| + | |||
| + | |||
| + | Nuclear fission of uranium-235 yields an enormous amount of energy from the fact that the fission products have less total mass than the uranium nucleus, a mass change that is converted to energy by the Einstein relationship <math>E=mc^2</math>. Using the Law of Conservation of Energy, we can look at the total energy before and after the fission to determine how much energy is released in this process. | ||
| + | |||
| + | <center><math>^{235}_{92}U : 218.8969\ MeV</math></center> | ||
| + | <center><math>^{140}_{54}Xe : \ MeV</math></center> | ||
Revision as of 03:12, 26 February 2019
When uranium-235 undergoes fission, the average of the fragment mass is about 118, but it is more probable that the pair will have an unequal distribution in mass. A common pair of fragments from uranium-235 fission is xenon and strontium as shown in the reaction:

Nuclear fission of uranium-235 yields an enormous amount of energy from the fact that the fission products have less total mass than the uranium nucleus, a mass change that is converted to energy by the Einstein relationship . Using the Law of Conservation of Energy, we can look at the total energy before and after the fission to determine how much energy is released in this process.