Difference between revisions of "ForestSolarCells"
| Line 26: | Line 26: | ||
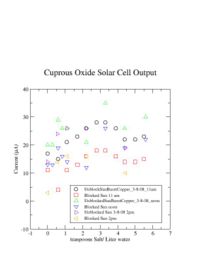
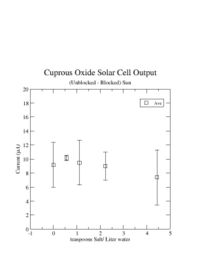
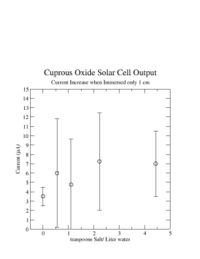
I was unable to observe a dependence of the solar cell output current on the amount of salt I added. | I was unable to observe a dependence of the solar cell output current on the amount of salt I added. | ||
| − | The standard setup for my project is a plastic bottle that contains hot or cold water, salt, and 2 sheets of copper. The alligator clips are attached to the micro ammeter. The micro ammeter measures how much current | + | The standard setup for my project is a plastic bottle that contains hot or cold water, salt, and 2 sheets of copper. One of the copper sheets was baked at a high temperature to convert the copper into cuprous oxide. The alligator clips are used to attached to the micro ammeter to the two copper sheets. The micro ammeter measures how much current flows between the two copper sheets. |
Revision as of 17:46, 9 March 2008
Does salt change the amount of current?
The materials I used were
1. A half square foot sheet of copper flashing.
2. Two alligator clip leads.
3. A sensitive micro-ammeter that can read currents between 10 and 50 microamperes.
4. An electric stove or oven.
5. A large clear plastic bottle with the top cut off. I used a 2 liter spring water bottle. A large mouth glass jar will also work.
6. Table salt.
7. Tap water.
8. Sand paper or a wire brush.
9. A Paper cutter for cutting the copper sheet.
Solar cells are designed to convert light into electrical energy. They do this without the use of either chemical reactions or moving parts. Modern solar cells are based on semiconductors, a material whose electrical properties lie between conductors and insulators. In a conductor electric current can flow freely. In a insulator electric current can't flow freely. Light hitting a semiconductor solar cell pushes electrons out of the semiconductor making an electric current we can use.
I was unable to observe a dependence of the solar cell output current on the amount of salt I added.
The standard setup for my project is a plastic bottle that contains hot or cold water, salt, and 2 sheets of copper. One of the copper sheets was baked at a high temperature to convert the copper into cuprous oxide. The alligator clips are used to attached to the micro ammeter to the two copper sheets. The micro ammeter measures how much current flows between the two copper sheets.
I learned that the amount of salt doesn't change the current.
A 6" x 6" copper flashing was baked in a 500 degree celsius oven for 20 Minutes.
The picture below shows the difference between the two burnt copper sheets. The sheet on the left was baked at 500 celsius and the one on the right was cooked on a stove top. You can see that the baked copper sheet has more of the red cuprous oxide visible while the one burnt on the stove top has the red cuprous oxide located mostly in the lower half of the sheet.
Aparatus:
Performance: