Difference between revisions of "Lead Shield Cone"
| Line 188: | Line 188: | ||
<math>\rho = \frac{p}{R_{\rm specific} T} </math> | <math>\rho = \frac{p}{R_{\rm specific} T} </math> | ||
| + | |||
| + | Using the normal temperature and pressure of 20 °C (293.15 K, 68 °F) and an absolute pressure of 1 atm (14.696 psi, 101.325 kPa) | ||
| + | |||
| + | |||
| + | <math>\rho = \frac{101.325 kPa}{335.505 \frac{J}{g \cdot K} 293.15 K} </math> | ||
Revision as of 16:56, 23 January 2018
Target
We need to edit the EXn02DetectorConstruction file to allow for a target that is a lead cone to simulate the Moller Shield. The header file for an elliptical Cone gives the parameters need to call with.
// $Id: G4EllipticalCone.hh 67011 2013-01-29 16:17:41Z gcosmo $ // // // -------------------------------------------------------------------- // GEANT 4 class header file // // G4EllipticalCone // // Class description: // // G4EllipticalCone is a full cone with elliptical base which can be cut in Z. // // Member Data: // // xSemiAxis semi-axis, x, without dimentions // ySemiAxis semi-axis, y, without dimentions // zheight height, z // zTopCut upper cut plane level, z // // The height in Z corresponds to where the elliptical cone hits the // Z-axis if it had no Z cut. Also the cone is centered at zero having a // base at zTopCut and another at -zTopCut. The semi-major axes at the Z=0 // plane are given by xSemiAxis*zheight and ySemiAxis*zheight so that the // curved surface of our cone satisfies the equation: // // *************************************************************************** // * * // * (x/xSemiAxis)^2 + (y/ySemiAxis)^2 = (zheight - z)^2 * // * * // *************************************************************************** // // In case you want to construct G4EllipticalCone from : // 1. halflength in Z = zTopCut // 2. Dx and Dy = halflength of ellipse axis at z = -zTopCut // 3. dx and dy = halflength of ellipse axis at z = zTopCut // ! Attention : dx/dy=Dx/Dy // // You need to find xSemiAxis,ySemiAxis and zheight: // // xSemiAxis = (Dx-dx)/(2*zTopCut) // ySemiAxis = (Dy-dy)/(2*zTopCut) // zheight = (Dx+dx)/(2*xSemiAxis)
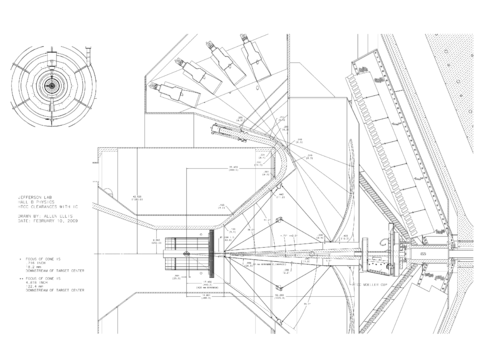
The geometry looks like
Solving the variables:
1. zTopCut
The cone shape starts 380 mm from the vertex point. It extends 1325.9 beyond the starting position. This gives the full length from where it would cross the z axis as 1705.9 mm. Since this cone is centered at the origin with zTopCut positive and negative placed an equal distance from the vertex, 1325.9/2=662.95 mm.
2. Dx and Dy
The length of the x and y components at the larger end of the cone are found from the radius at this position. Using geometry, for a right triangle with it's apex at the vertex and a height of 1705.9, with an interior angle of 5 degrees.
3. dx and dy
Using geometry, for a right triangle with it's apex at the vertex and a height of 380, with an interior angle of 5 degrees.
Solving for the variable to pass to the function:
The height in z is defined at the the point where the cone vertex crosses the z=0 plane in x&y. From the drawing, the vertex is originally placed at 0,0,0. Since the GEANT4 cone is centered at a new vertex with the circular cross sections of the cone at +/- 662.95, and the distance from the first smaller cross section is 380, this gives the intersection at 1042.95 cm with respect to the new vertex. Verifying this:
NOTE: This will need to be rotated 180 degrees so that the large end is nearest to the particle vertex.
Particle Gun
mkdir ParticleGunInputs
root -l LUND_Spread_LH2_IsotropicPhi.C
split -d -l 3000 -a 3 LUND_Spread_LH2_IsotropicPhi.LUND LUND_Spread_LH2_IsotropicPhi_
prename 's/(LUND_Spread_LH2_IsotropicPhi_\d{3})/$1.LUND/' LUND_Spread_LH2_IsotropicPhi_*
Using the file LUND2ParticleGun.C 106 macros for the GEANT4 ParticleGun are created that mirror the LUND files. The same random number is initially input for each macro as was used for the LUND file.
Running Simulations
Moller Shield effects
Event # 902
Step# X Y Z Px Py Pz KineE dEStep StepLeng TrakLeng Volume Process Theta(pos) theta(mom) Phi(pos)
0 0 fm 0 fm -2 m 9.61695 keV 5.55235 keV 122.019 keV 14.48357 keV 0 eV 0 fm 0 fm World initStep NA NA NA
1 10.09023 cm 5.825597 cm -71.97609 cm 9.61695 keV 5.55235 keV 122.019 keV 14.48357 keV 4.258733e-16 eV 1.28553 m 1.28553 m World Transportation 5.2 5.2 30
2 10.09023 cm 5.825597 cm -71.97609 cm -31.23412 keV 91.41135 keV 72.1564 keV 14.03255 keV 451.0172 eV 49.21704 nm 1.28553 m Target msc 5.2 53.24 108.9
3 10.09023 cm 5.825598 cm -71.97609 cm -59.67862 keV 79.92509 keV 67.73916 keV 14.03255 keV 0 eV 2.216658 nm 1.28553 m Target Transportation 5.2 55.82 126.7
4 10.09023 cm 5.825598 cm -71.97609 cm -59.67862 keV 79.92509 keV 67.73916 keV 14.03255 keV 0 eV 0 fm 1.28553 m World Transportation 5.2 55.82 126.7
5 10.09022 cm 5.825604 cm -71.97608 cm -0 eV 0 eV 0 eV 0 eV 14.03255 keV 1.171968 um 1.285531 m Target eIoni 5.2 -nan -nan
Event # 903
Step# X Y Z Px Py Pz KineE dEStep StepLeng TrakLeng Volume Process Theta(pos) theta(mom) Phi(pos)
0 0 fm 0 fm -2 m 9.43847 keV -5.4493 keV 117.483 keV 13.44453 keV 0 eV 0 fm 0 fm World initStep NA NA NA
1 15.99911 cm -9.237085 cm -8.55066 mm 9.43847 keV -5.4493 keV 117.483 keV 13.44453 keV 7.06826e-16 eV 2 m 2 m World Transportation 5.3 5.3 -30
2 16.00511 cm -9.240549 cm -7.803867 mm 9.43847 keV -5.4493 keV 117.483 keV 13.44453 keV 2.650598e-19 eV 750 um 2.00075 m Tracker StepLimiter 5.3 5.3 -30
The Moller particles interact with the target up to 5.3 degrees in Theta.
Effects of DC/Tracker on Moller particles
The DC chamber is filled with a mixture of 90%/10% of Ar/CO2. The density of this gas can be calculated can be calculated using the ideal gas law, expressed as a function of temperature and pressure:
where:
The specific gas constant of a gas or a mixture of gases () is given by the molar gas constant divided by the molar mass (M) of the gas or mixture, or in turn by Boltzmann constant over the molecular mass of the gas.
The average molar mass of mixtures can be calculated from the mole fractions of the components and their molar masses :
M(Ar) = 39.948 × 1 g/mol = 39.948 g/mol M(CO2) = 12.01+16.00*2× 1 g/mol = 44.01 g/mol
where:
R=8.314 J / mol. K.
Using the normal temperature and pressure of 20 °C (293.15 K, 68 °F) and an absolute pressure of 1 atm (14.696 psi, 101.325 kPa)