Difference between revisions of "Forest He-3 Tubes"
| Line 7: | Line 7: | ||
<math>p+He^3 \rightarrow p + He^3(+) + e^-</math> | <math>p+He^3 \rightarrow p + He^3(+) + e^-</math> | ||
| − | The same proton will ionize several He-3 atoms when dissipating the 573 keV kinetic energy. | + | The same proton will ionize several He-3 atoms when dissipating the 573 keV kinetic energy. Once you have the creation of ions, you can construct detectors to collect and measure the electrons. |
The Tritium (H^3) can also ionize the gas but due to its higher mass it does not travel as far (shorter range) as the proton and makes a smaller contribution to the ionization signal. Tritium decays to He-3 after about 12 years ( neutron is converted to proton) | The Tritium (H^3) can also ionize the gas but due to its higher mass it does not travel as far (shorter range) as the proton and makes a smaller contribution to the ionization signal. Tritium decays to He-3 after about 12 years ( neutron is converted to proton) | ||
| Line 19: | Line 19: | ||
The probability of neutron capture is measured in terms of a cross section. There is a nuclear data base for neutron capture located at [http://ie.lbl.gov/ng.html LBL]. The "free" neutron thermal cross section is [http://ie.lbl.gov/ngdata/sig.htm <math>3.10 \pm 0.13</math>] barns ( 1 barn = <math>10^{-24} cm^2</math>). | The probability of neutron capture is measured in terms of a cross section. There is a nuclear data base for neutron capture located at [http://ie.lbl.gov/ng.html LBL]. The "free" neutron thermal cross section is [http://ie.lbl.gov/ngdata/sig.htm <math>3.10 \pm 0.13</math>] barns ( 1 barn = <math>10^{-24} cm^2</math>). | ||
| + | |||
| + | |||
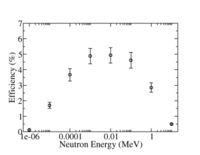
10-atm He-3, 2.54 cm diameter tube, 76 cm long, poly moderator, cadmium metal, Boron loaded shielding. | 10-atm He-3, 2.54 cm diameter tube, 76 cm long, poly moderator, cadmium metal, Boron loaded shielding. | ||
Revision as of 19:33, 7 June 2008
Thermal neutron capture of He-3 may be represented by the reaction below
About 764 keV of energy is liberated in this nuclear reaction and distributed between the final products according to their masses. Because the proton is about a factor of 3 lighter than Tritium (H^3) , it will have more kinetic energy by about a factor of 3 (about 573 keV). This liberated proton can ionize other He-3 atoms via the reaction
The same proton will ionize several He-3 atoms when dissipating the 573 keV kinetic energy. Once you have the creation of ions, you can construct detectors to collect and measure the electrons.
The Tritium (H^3) can also ionize the gas but due to its higher mass it does not travel as far (shorter range) as the proton and makes a smaller contribution to the ionization signal. Tritium decays to He-3 after about 12 years ( neutron is converted to proton)
reference: J. W. Leake, "Nuclear Instruments and Methods", Vol. 63, page 329, 1968).
The probability of neutron capture is measured in terms of a cross section. There is a nuclear data base for neutron capture located at LBL. The "free" neutron thermal cross section is barns ( 1 barn = ).
10-atm He-3, 2.54 cm diameter tube, 76 cm long, poly moderator, cadmium metal, Boron loaded shielding.