Difference between revisions of "LB Thesis Outline"
(→Theory) |
|||
| (22 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
=Abstract= | =Abstract= | ||
| − | Photon Activation Analysis | + | Photon Activation Analysis (PAA) is a technique used to measure nuclear isotopic concentrations by observing the characteristic gamma decay of a sample of interest post-irradiation. The photons used to irradiate a sample were produced by accelerating electrons to an energy of 32 MeV at the Idaho Accelerator Center and impinging those electrons onto a Tungsten/Aluminum converter to create Bremsstrahlung radiation. Four soil samples, with varying concentrations of Selenium by mass (50%, 10%, 1%, 0.1%), were irradiated. The purpose of the experiment was to determine the minimum concentration in which Se-81m and Se-75 can be accurately identified when embedded in a soil matrix. This minimum observable concentration, referred to as the minimum detectable limit, was measured in term of the signal to noise ratios of the 103 keV gamma decay energy from Se-81m and the 265 keV gamma decay energy from Se-75. The detection limit of Selenium in soil using PAA will be reported. |
=Theory= | =Theory= | ||
| Line 6: | Line 6: | ||
=Experimental Setup= | =Experimental Setup= | ||
| + | |||
| + | Give beam parameters, include schematic of the experimental setup (xfig),include pictures of accelerator and W/Al Radiator. Give GPS coordinates for soil gathered. | ||
==Target Information== | ==Target Information== | ||
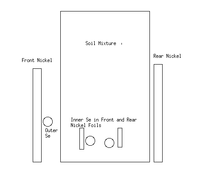
| + | a schematic of the target is shown below: | ||
| + | |||
| + | [[File:LB TargetSetupxFig.png|200px]] | ||
| + | |||
| + | {| border="3" cellpadding="5" cellspacing="0" | ||
| + | |Sample || Soil Mass (g) || Inner Se Mass (g) || Outer Se Mass (g) || Front Outer Nickel Foil (g) || Front Inner Nickel Foil (g) || Rear Inner Nickel Foil (g) || Rear Outer Nickel Foil (g) | ||
| + | |- | ||
| + | ||50% Sample || 0.4921 || 0.5145 || 0.0900 || 1.5535 || 0.3956 || 0.3050 || 1.6140 | ||
| + | |- | ||
| + | ||10% Sample || 4.9921 || 0.5142 || 0.1245 || 1.3310 || 0.3592 || 0.2768 || 1.2196 | ||
| + | |- | ||
| + | ||1% Sample || 10.2403 || 0.0954 || 0.0914 || 3.4274 || 1.8672 || 1.7220 || 3.4887 | ||
| + | |- | ||
| + | || 0.1% Sample || 100.1242 || 0.1074 || 0.1042 || 1.3911 || 0.4467 || 0.2548 || 1.3804 | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | ==DAQ== | ||
| + | [[LB Thesis DAQ Writeup]] | ||
| + | |||
| + | ==Detector Efficiencies== | ||
| + | [[LB May 2017 Det A Efficiency]] | ||
| + | |||
| + | [[LB May 2017 Det B Efficiency]] | ||
| + | |||
| + | |||
| + | ==Calibration Method== | ||
| + | When using the HpGe detector, the energy isn't directly given due to the fact that the energy deposited by the photon into the crystal is converted into an ADC channel number. The energy calibration is quite simple. By using calibrated sources with known activities, dates of activation, and energy lines, one can place the calibrated source in front of the HpGe detector and find which channel number corresponds to the energy line of interest. This would be a single data point in your calibration. To ensure that the calibration is of a high quality, take several sources with several different energy lines that span the channel range of the detector and compare the channel number to the energy. Once this information has been found, simply make a file with the energy the line was supposed to be, the channel number, and the standard deviation from a Gaussian fit and plot the points. Once the points are plotted a linear fit can be done to find exactly how the energy lines deviate from the raw channel number. | ||
=Data/Error Analysis= | =Data/Error Analysis= | ||
| + | [[LB Thesis Thin Window Analysis]] | ||
| + | |||
| + | |||
| + | ==Split Run Measurement Technique== | ||
| + | Since there were other people present on the day of irradiation that were using the detectors, a more creative measurement approach was devised. It was known that potentially only 1 detector would be available for use, so a flagging system was implemented to distinguish how the sample type was changed over the time of the measurement. A Co-60 flag was used because it only has energy lines well above the selenium's lines of interest. This means that there would be no extra signal near the region of interest while taking data. For example, a run would be started on a detector with a pure selenium sample being counted. After some amount of time (usually a minimum of 5 minutes) the pure selenium sample was removed and the Co-60 flag was placed in front of the detector for roughly 1 minute to ensure that the Co-60 would be visible. It is worth noting that the time here is important as the detector has a much lower efficiency near the Co-60 lines, so a longer Co-60 measurement is advised to ensure a signal. Once the Co-60 lines are clearly visible in the spectrum, switch the Co-60 out for the selenium-soil mixture and continue counting. Once the mixture is done counting, reintroduce the Co-60 for roughly 1 minute, then swap it out with the pure selenium sample again. This can be repeated for the length of the entire runtime. | ||
=Conclusion= | =Conclusion= | ||
| + | |||
| + | =Appendices= | ||
| + | *Include arrays of plots similar to analysis pages? | ||
| + | |||
| + | *Include ROOT macros? | ||
| + | |||
| + | *Include runlists? | ||
Latest revision as of 21:07, 30 May 2018
Abstract
Photon Activation Analysis (PAA) is a technique used to measure nuclear isotopic concentrations by observing the characteristic gamma decay of a sample of interest post-irradiation. The photons used to irradiate a sample were produced by accelerating electrons to an energy of 32 MeV at the Idaho Accelerator Center and impinging those electrons onto a Tungsten/Aluminum converter to create Bremsstrahlung radiation. Four soil samples, with varying concentrations of Selenium by mass (50%, 10%, 1%, 0.1%), were irradiated. The purpose of the experiment was to determine the minimum concentration in which Se-81m and Se-75 can be accurately identified when embedded in a soil matrix. This minimum observable concentration, referred to as the minimum detectable limit, was measured in term of the signal to noise ratios of the 103 keV gamma decay energy from Se-81m and the 265 keV gamma decay energy from Se-75. The detection limit of Selenium in soil using PAA will be reported.
Theory
Experimental Setup
Give beam parameters, include schematic of the experimental setup (xfig),include pictures of accelerator and W/Al Radiator. Give GPS coordinates for soil gathered.
Target Information
a schematic of the target is shown below:
| Sample | Soil Mass (g) | Inner Se Mass (g) | Outer Se Mass (g) | Front Outer Nickel Foil (g) | Front Inner Nickel Foil (g) | Rear Inner Nickel Foil (g) | Rear Outer Nickel Foil (g) |
| 50% Sample | 0.4921 | 0.5145 | 0.0900 | 1.5535 | 0.3956 | 0.3050 | 1.6140 |
| 10% Sample | 4.9921 | 0.5142 | 0.1245 | 1.3310 | 0.3592 | 0.2768 | 1.2196 |
| 1% Sample | 10.2403 | 0.0954 | 0.0914 | 3.4274 | 1.8672 | 1.7220 | 3.4887 |
| 0.1% Sample | 100.1242 | 0.1074 | 0.1042 | 1.3911 | 0.4467 | 0.2548 | 1.3804 |
DAQ
Detector Efficiencies
Calibration Method
When using the HpGe detector, the energy isn't directly given due to the fact that the energy deposited by the photon into the crystal is converted into an ADC channel number. The energy calibration is quite simple. By using calibrated sources with known activities, dates of activation, and energy lines, one can place the calibrated source in front of the HpGe detector and find which channel number corresponds to the energy line of interest. This would be a single data point in your calibration. To ensure that the calibration is of a high quality, take several sources with several different energy lines that span the channel range of the detector and compare the channel number to the energy. Once this information has been found, simply make a file with the energy the line was supposed to be, the channel number, and the standard deviation from a Gaussian fit and plot the points. Once the points are plotted a linear fit can be done to find exactly how the energy lines deviate from the raw channel number.
Data/Error Analysis
LB Thesis Thin Window Analysis
Split Run Measurement Technique
Since there were other people present on the day of irradiation that were using the detectors, a more creative measurement approach was devised. It was known that potentially only 1 detector would be available for use, so a flagging system was implemented to distinguish how the sample type was changed over the time of the measurement. A Co-60 flag was used because it only has energy lines well above the selenium's lines of interest. This means that there would be no extra signal near the region of interest while taking data. For example, a run would be started on a detector with a pure selenium sample being counted. After some amount of time (usually a minimum of 5 minutes) the pure selenium sample was removed and the Co-60 flag was placed in front of the detector for roughly 1 minute to ensure that the Co-60 would be visible. It is worth noting that the time here is important as the detector has a much lower efficiency near the Co-60 lines, so a longer Co-60 measurement is advised to ensure a signal. Once the Co-60 lines are clearly visible in the spectrum, switch the Co-60 out for the selenium-soil mixture and continue counting. Once the mixture is done counting, reintroduce the Co-60 for roughly 1 minute, then swap it out with the pure selenium sample again. This can be repeated for the length of the entire runtime.
Conclusion
Appendices
- Include arrays of plots similar to analysis pages?
- Include ROOT macros?
- Include runlists?